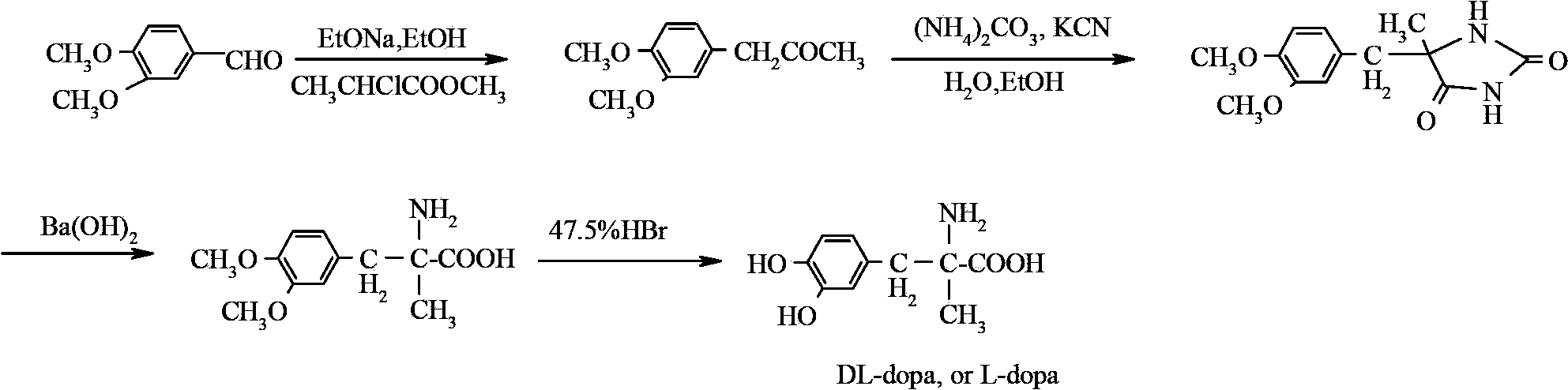
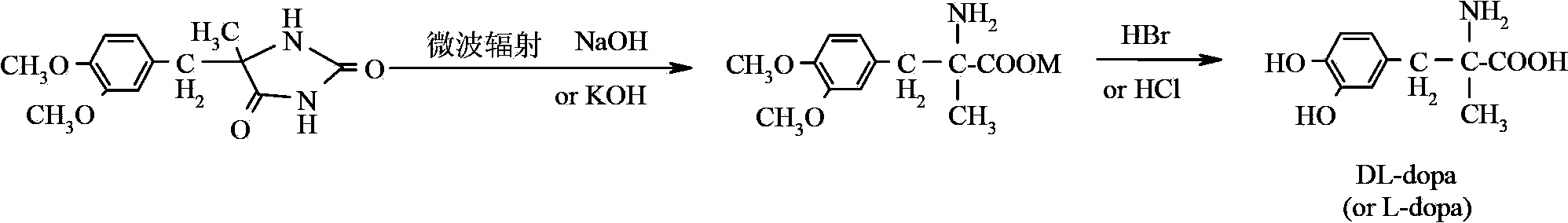
Method for preparing methyldopa through microwave basic hydrolysis 5-methyl-5-(3,4-dimethoxy benzyl) hydantoin
A technology of dimethoxybenzyl and dimethoxyphenyl, which is applied to the preparation of methyl polymethylpolyphenol by microwave alkali hydrolysis of 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin In the field of Pakistan, it can solve problems such as the difficulty in hydrolyzing NaOH lye
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]In the reaction flask, add 4.3g (0.016mol) of 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin, and place in a solution of 2.1gNaOH (0.053mol) dissolved in 17ml of water , the reaction mixture was placed in a chemical experiment temperature-controlled microwave instrument, and irradiated for 15 minutes at a microwave power of 250 watts. After cooling, adjust the pH to 4 with 6N hydrochloric acid, filter under reduced pressure to obtain a solid, wash with 2x5ml of ethanol, then dissolve in about 30-40ml of acetone, adjust the pH to 8.8 with ammonia water, and crystallize. Carefully add a small amount of glacial acetic acid to adjust the pH to 6, and keep the pH stable, then filter and separate to obtain the product DL-α-methyl-(3,4-dimethoxyphenyl)-α-alanine, and vacuum-dry at 80°C Dry. Yield 73.7%.
Embodiment 2
[0029] In the reaction flask, add 4.3g (0.016mol) 5-methyl-5-(3,4-dimethoxybenzyl) hydantoin, put 2.56gNaOH (0.064mol) in 17ml water solution , irradiate for 15 minutes at a microwave power of 250 watts. After cooling, adjust the pH to 4 with 6N hydrochloric acid, filter under reduced pressure to obtain a solid, wash with 2x5ml of ethanol, then dissolve in about 30-40ml of acetone, adjust the pH to 8.8 with ammonia water, and crystallize. Carefully add a small amount of glacial acetic acid to adjust the pH to 6, and keep the pH stable, then filter and separate to obtain the product DL-α-methyl-(3,4-dimethoxyphenyl)-α-alanine, and vacuum-dry at 80°C Dry. Yield 78.7%.
Embodiment 3
[0031] In the reaction flask, add 4.3g (0.016mol) 5-methyl-5-(3,4-dimethoxybenzyl) hydantoin, put 2.56gNaOH (0.064mol) in 17ml water solution Medium, 500 Wh, irradiated for 10 minutes. After cooling, adjust the pH to 4 with 6N hydrochloric acid, filter under reduced pressure to obtain a solid, wash with 2x5ml of ethanol, then dissolve in about 30-40ml of acetone, adjust the pH to 8.8 with ammonia water, and crystallize. Carefully add a small amount of glacial acetic acid to adjust the pH to 6, and keep the pH stable, then filter and separate to obtain the product DL-α-methyl-(3,4-dimethoxyphenyl)-α-alanine, and vacuum-dry at 80°C Dry. Yield 83.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com