Anaesthetic formulation
A technology of anesthetics and anesthetics, applied in anesthetics, drug combinations, pharmaceutical formulations, etc., can solve problems such as increased injection pain, inhibition, and complexity of syringe delivery equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0108] Anesthesia of alfaxalone in 30% w / v (7) sulfobutyl ether β-cyclodextrin
[0109] Alfa Salon is formulated as 6ml of clear colorless liquid which contains:
[0110] Alfa Salon 60mg (10mg / ml);
[0111] (7) 1800 mg of sulfobutyl ether β-cyclodextrin;
[0112] Saline (0.9% w / v) 6ml.
[0113] This is the molar compounding ratio of alfasalone and (7) sulfobutyl ether β-cyclodextrin 1:4.6. Male Wistar rats (weight [wt] 270-315 g) with an indwelling catheter in the jugular vein were placed in a Perspex holder and given injections, the accompanying observations are shown in Table 1 upon release from the holder.
[0114] Table 1
[0115] Effects of Alfaxalon Preparation on Wistar Rats
[0116]
[0117] KEY
[0118]
[0119] Rats given 25 and 100 mg / kg body weight recovered over 60 minutes; they did not die or suffer any adverse effects at these doses. In these experiments it was seen that intravenous administration of alfaxalone at 10 mg / ml dissolved in (7) sulfobuty...
example 2
[0121] Pharmacokinetics in Rats
[0122] Two groups of 10 rats implanted with internal jugular vein intravenous catheter and carotid arterial catheter received intravenous injection of 10 mg / kg body weight of Alfasalone (7) sulfobutyl ether β-cyclodextrin preparation via jugular vein (n=10 rats) or a mixture of alfaxalone and alfadolone in Cremophor EL (polyoxyethylene castor oil) at 1.1 ml / kg (n=10 rats). Blood drawn from the carotid artery or tail at various time intervals after this injection was analyzed for alfaxalon blood levels. These blood levels were fitted to a three-compartment pharmacokinetic model and means±sem of key parameters were calculated for both steroidal anesthetic formulations.
[0123] It is expected that there will be no significant differences between the calculated PK parameters for (7) sulfobutyl ether β-cyclodextrin formulation and Althesin (registered trademark) formulation. This would suggest that the new formulation is expected to work in a si...
example 3
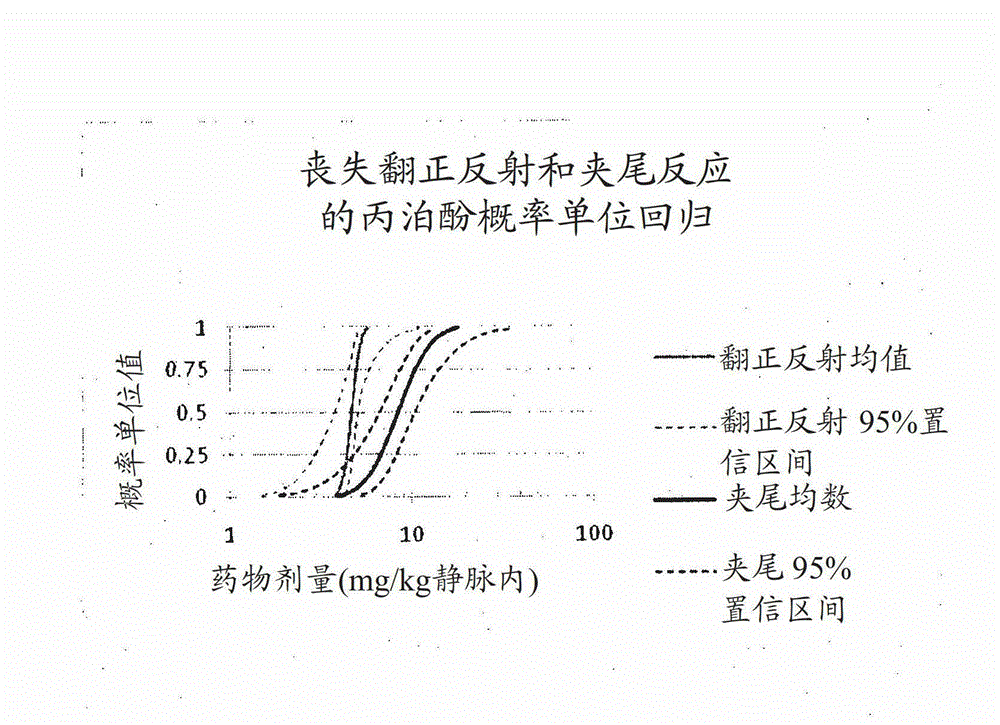
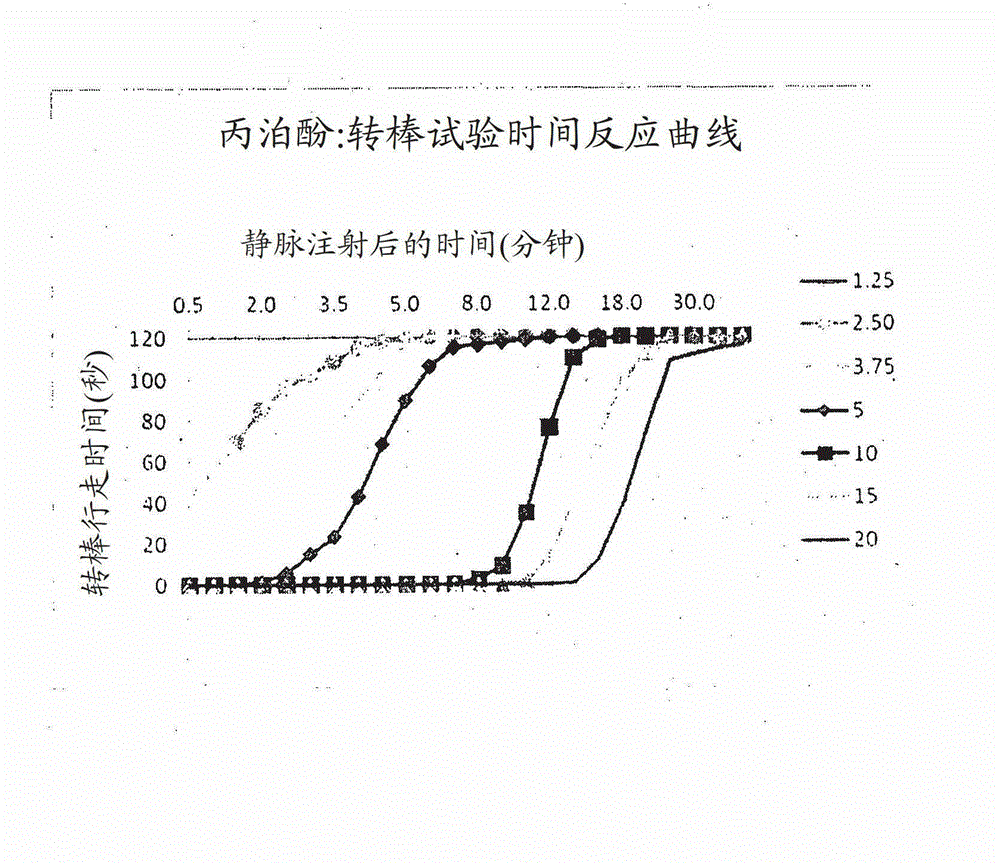
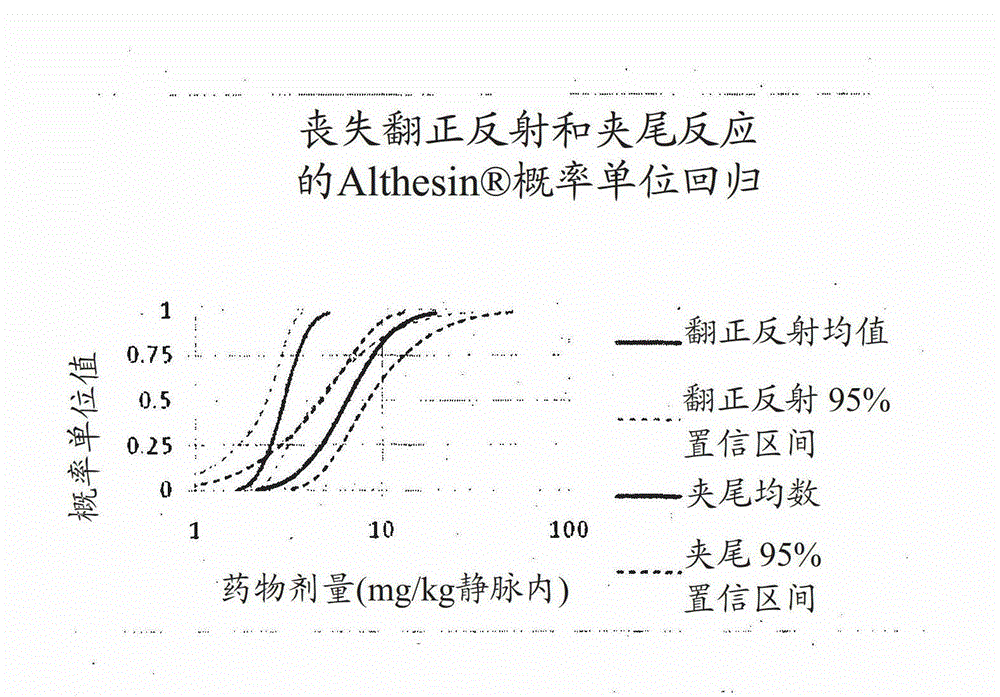
[0128] Anesthetic effect of alfaxalone in (7) sulfobutyl ether β-cyclodextrin compared with alfaxalone and propofol as Althesin (registered trademark)
[0129] Male Wistar rats (wt 150-220 g) were implanted with an indwelling intravenous catheter in the internal jugular vein under halothane anesthesia. After 24 hours, each rat received intravenous injections in the following dose ranges: Propofol (10 mg / ml in 10% w / v Intralipid emulsion; Diprivan [registered trademark]); Althesin [registered trademark] ( Alfaxalone in 20% w / v CremophorEL 9 mg / ml plus alfadrolone acetate 3 mg / ml); or Phaxan CD (Alfaxalone 10 mg / ml in a 1:2 molar complex with Captisol (registered trademark)-(7)sulfobutyl ether β-cyclodextrin). The following were assessed at regular intervals after the intravenous infusion:
[0130] 1. Righting Reflex: Score: 1 Normal; 2 Slow; 3 Some Attempt; 4 None - This is a measure of the onset and duration of loss of consciousness;
[0131] 2. Tail pinch reaction: scored ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com