Cell penetrating peptide hPP10 and use thereof
A technology of penetrating peptides and cells, which is applied in the field of biomedicine to achieve the effects of little possibility, few unsafe factors, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1, CPP primary structure, secondary structure analysis, prediction and identification of new human-derived CPP:
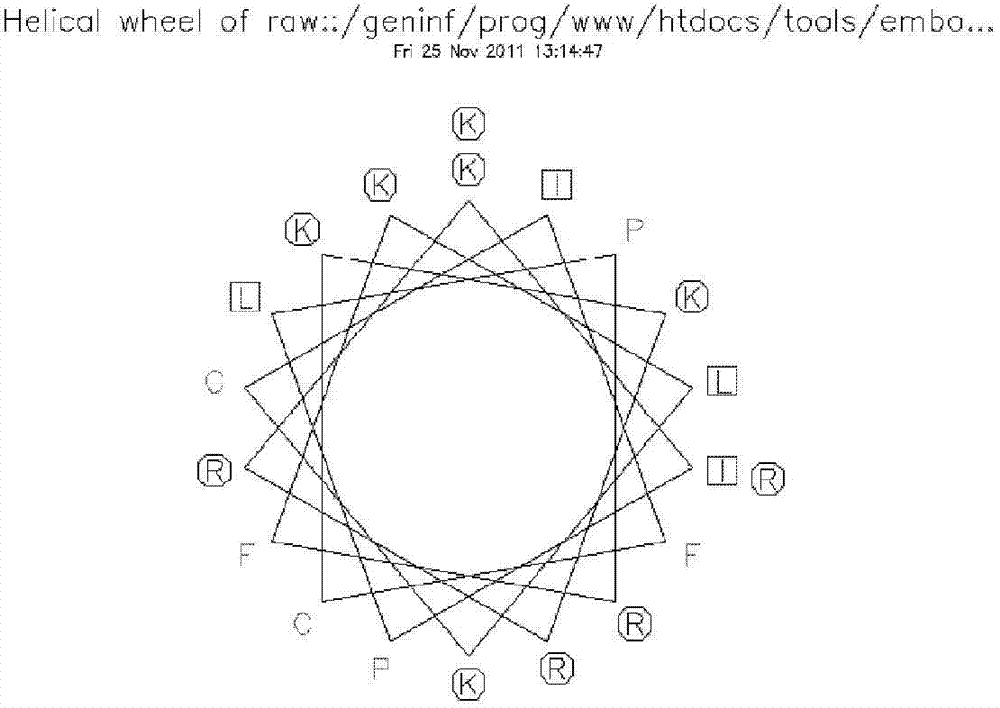
[0052] The secondary structure analysis uses the online analysis program of emboss (for the analysis program, please refer to the webpage: http: / / emboss.bioinformatics.nl / cgi-bin / emboss / pepwheel online analysis of the wheel structure of peptides; http: / / emboss. bioinformatics.nl / cgi-bin / emboss / online analysis of secondary structures (helices, folds, etc.). The schematic diagram of the wheel structure of hPP10, the schematic diagrams of the helical and folded structures are as follows figure 1 and figure 2 shown.
[0053] Chemical synthesis of green fluorescent labeled hPP10:
[0054] FITC-
[0055] Lys-Ile-Pro-Leu-Pro-Arg-Phe-Lys-Leu-Lys-Cys-Ile-Phe-Cys-Lys-Lys-Arg-Arg-Lys-Arg (hPP10-FITC)
[0056] and hPP10 without green fluorescent labeling:
[0057] Lys-Ile-Pro-Leu-Pro-Arg-Phe-Lys-Leu-Lys-Cys-Ile-Phe-Cys-Lys-Lys-Arg-Arg-Lys-Arg(hPP10)
...
Embodiment 2
[0121] Example 2, hPP10-mediated plasmid DNA transfection
[0122] The method of hPP10-mediated plasmid DNA transfection includes the following steps: (1) culture ECV-304 cells in the logarithmic growth phase, and use 1×10 5 The seeding density of cells / well was inoculated in a 12-well plate for routine culture, and corresponding positive and negative wells were set at the same time;
[0123] (2) Set at 37°C 5% CO 2 In the incubator, cultivate for 20~24h, and when the cell density reaches 70%-80%, replace the culture medium with serum-free RPMI-1640 culture medium, and continue to cultivate for 1 hour;
[0124] (3) Meanwhile prepare transfection samples:
[0125] a. Take a final concentration of 10 μM hPP10 and add it to a centrifuge tube containing 50 μl Opti-MEM, mix gently and incubate at room temperature for 5 minutes; b. Take 0.8 μg of the plasmid and add it to another centrifuge tube containing 50 μl Opti-MEM, Mix gently and incubate at room temperature for 5 min. Sl...
Embodiment 3
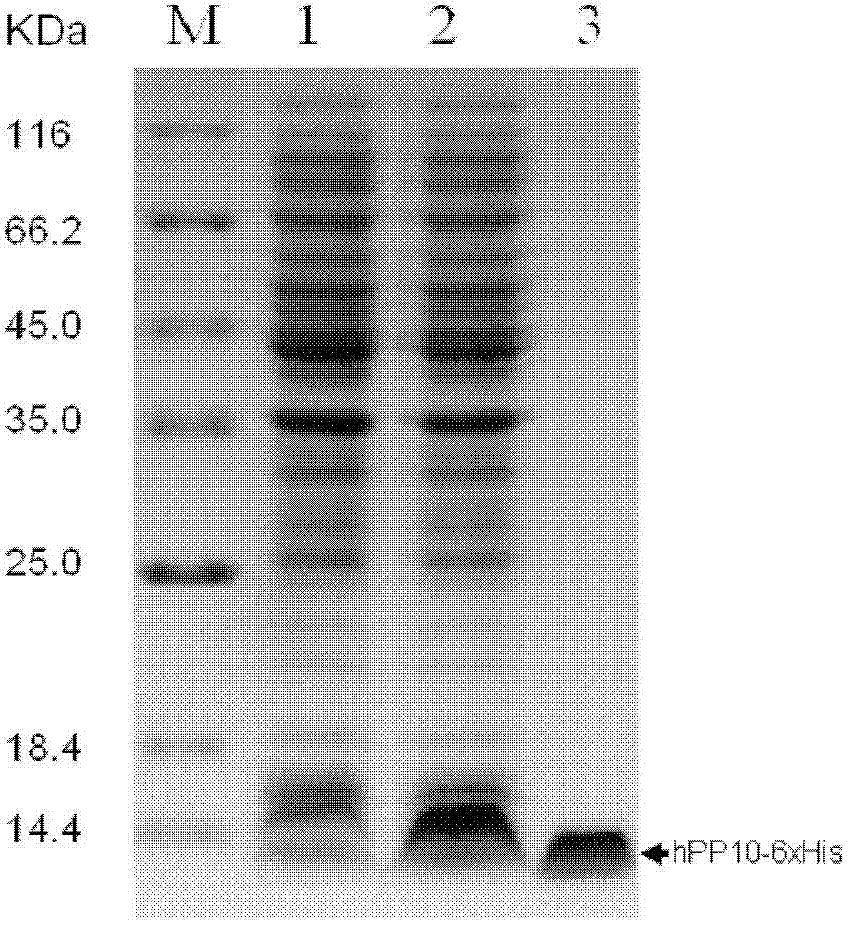
[0147] Example 3. Construction of pET15b-hPP10-GFP plasmid, expression and purification of fusion protein and research on its transmembrane efficacy
[0148] 3.1 Construction and identification of pET15b-hPP10-GFP recombinant plasmid
[0149] (1) Design two single-stranded cDNAs encoding hPP10, with NdeI and XhoI restriction sites on both sides, and submit them to Shanghai Shenggong Company to synthesize single-stranded oligonucleotide chains, and then mix the two single-stranded DNAs in equal amounts Add it into an aqueous solution, and cool it down to room temperature naturally for 5 minutes at 95°C to complete annealing to form a complementary double-stranded DNA fragment (hPP10); meanwhile, design a pair of primers and use pEGFP (purchased from Clontech) as a template to obtain GFP by PCR Protein gene fragment, with XhoI and BamHI restriction sites on both sides, purified PCR product for future use;
[0150] (2) Carry out double digestion with two restriction endonuclease...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com