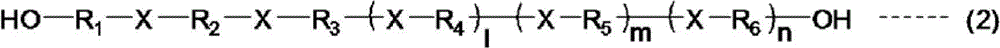Active energy ray curable resin composition, cured product thereof, and film
A technology of active energy rays and curable resin, which is applied in the direction of coating, etc., can solve the problems of unable to get fingerprints, hinder the curing of the coating film surface, and not easy to be conspicuous, etc., and achieve the effect of high surface hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
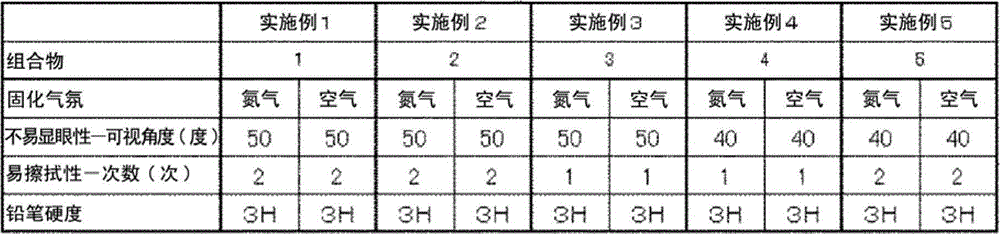
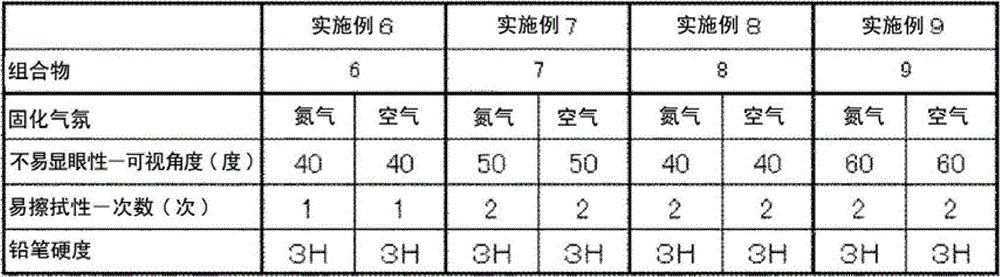
[0082] Examples and comparative examples are shown below, and the present invention will be described in more detail. Below, unless otherwise stated, "parts" and "%" are mass standards.
[0083] In addition, in the examples of the present invention, weight average molecular weight (Mw) and number average molecular weight (Mn) were measured using gel permeation chromatography (GPC) under the following conditions.
[0084] Measuring device: HLC-8220GPC manufactured by Tosoh Corporation
[0085] Column: TSK-GUARDCOLUMN SuperHZ-L manufactured by Tosoh Corporation+TSK-GEL SuperHZM-M manufactured by Tosoh Corporation×4
[0086] Detector: RI (differential refractometer)
[0087] Data processing: Multistation GPC-8020modelII manufactured by Tosoh Corporation
[0088] Determination conditions: column temperature 40°C
[0089] Solvent Tetrahydrofuran
[0090] Flow rate 0.35ml / min
[0091] Standard: Monodisperse Polystyrene
[0092] Sample: A sample (100 μl) obtained by filtering ...
Synthetic example 1
[0093] Synthesis Example 1 [Synthesis of Urethane Acrylate (A)]
[0094] 4000 g (2 moles) of polypropylene glycol with a weight average molecular weight (Mw) of 2000 and 91 g (1 mole) of 1,2-bis(2-hydroxyethylthio)ethane were charged into the flask, and octane was added as a catalyst. Tin (II) and zinc octanoate (II) each 200ppm, dibutyl hydroxytoluene 3000ppm as a polymerization inhibitor, p-methoxyphenol 300ppm, n-butyl acetate as a solvent so that the solid content in the flask is 80% and mix well, adjust the temperature in the system to 50°C. Thereafter, 261 g (3 mol) of toluene diisocyanate was divided into three parts and added while paying attention to heat generation, and it was made to react at 80 degreeC for 1 hour. Furthermore, 260 g (2 mol) of hydroxypropyl acrylates were added, and it was made to react at 80 degreeC blowing in air until the isocyanate group disappeared completely, and the urethane acrylate (A1) of 24000 weight average molecular weight (Mw) was ob...
Synthetic example 11
[0114] Synthesis example 11 [synthesis of polyfunctional (meth)acrylate (B)]
[0115] 535.5 g of a mixture (weight ratio 60 / 40) of pentaerythritol triacrylate and pentaerythritol tetraacrylate was placed in the flask. Add 200ppm each of tin (II) octanoate and zinc (II) octanoate as a catalyst, 3000ppm dibutyl hydroxytoluene as an antioxidant, and 300ppm p-methoxyphenol as a polymerization inhibitor in the flask, and then mix n-Butyl acetate was used so that the solid content would be 80%, and the temperature in the system was adjusted to 50°C.
[0116] While blowing air into the system, 84 g of hexamethylene diisocyanate was added in three portions. The temperature in the system was raised to 80° C., and it was reacted at 80° C. until the isocyanate group in the system completely disappeared to obtain urethane acrylate (B1). The weight average molecular weight of the urethane acrylate (B1) analyzed by GPC was 1400. In addition, the acryloyl equivalent was 109 g / mol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com