Nitrogen monoxide donor-type oridonin 1,4-hydroxyl-modified derivative, and its preparation method and application
A technology of oridonin A and nitric oxide, applied in the field of natural medicine and medicinal chemistry, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
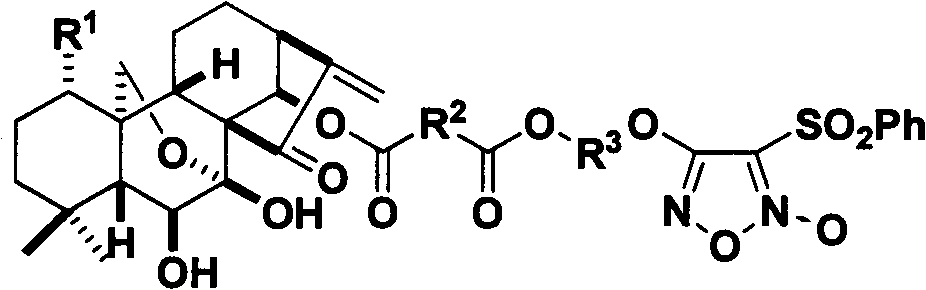
[0159] ent-1α,6β,7β-trihydroxy-(14β-O-(2-formylbenzoic acid-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4)-oxygen Ethyl ester))-15-oxo-7,20-oxo-16-kaurene
[0160] The compound 4-(2-(2-carboxybenzoyloxy)ethoxy)-3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide was dissolved in anhydrous dichloromethane, Add oridonin (72mg, 0.2mmol), EDC (93mg, 0.6mmol) and a catalytic amount of DMAP, and stir at room temperature for 12h. Wash with water, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography (dichloromethane:methanol=200:1) to obtain 54mg of white solid (yield 53.2%): mp.123-125°C; IR(KBr)υ max 3392, 2951, 2025, 1714, 1618, 1553, 1451, 1363, 739, 685cm -1 ; 1 H NMR (CDCl 3 , 300MHz), δ (ppm) 1.09 (6H, s, -CH 3 ), 3.37 (1H, d, J=9.6Hz, 13-CH), 3.50 (1H, m, 1-CH), 3.72 (1H, m, 6-CH), 4.05 (1H, s, 1-OH) , 4.07, 4.36 (each 1H, dd, J A =J B =10.2HZ, 20-CH 2 ), 4.67 (2H, m, -CH 2 ), 4.76 (2H, m, -CH 2 ), 5.54 (1H, s, 17-CH 2 ), 6.04 (1H, d, J=12.0Hz,...
Embodiment 2
[0162] ent-1α,6β,7β-trihydroxy-(14β-O-(2-formylbenzoic acid-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4)-oxygen Propyl ester))-15-oxo-7,20-oxo-16-kaurene
[0163] With reference to the synthetic method of Example 1, 52 mg (yield 47.6%) of white solid was obtained: mp.113-115 ° C; IR (KBr) υ max 3418, 2954, 2025, 1714, 1616, 1554, 1451, 1384, 736, 685cm -1 ; 1 H NMR (CDCl 3 , 300MHz), δ (ppm) 1.11 (6H, s, -CH 3 ), 3.39 (1H, d, J=9.9Hz, 13-CH), 3.50 (1H, m, 1-CH), 3.76 (1H, m, 6-CH), 4.06 (1H, s, 1-OH) , 4.08, 4.34 (each 1H, dd, J A =J B =10.2Hz, 20-CH 2 ), 4.46 (1H, m, -CH 2 ), 4.57 (1H, m, -CH 2 ), 4.58 (2H, m, -CH 2 ), 5.56 (1H, s, 17-CH 2 ), 6.05 (1H, d, J=10.5Hz, 6-OH), 6.07 (1H, s, 14-CH), 6.17 (1H, s, 17-CH 2 ), 7.52 (2H, m, Ar-H), 7.57 (3H, m, Ar-H), 7.75 (2H, m, Ar-H), 8.06 (2H, d, J=7.5Hz, Ar-H) ; MS (ESI) m / z: 812.3 [M+NH 4 ] + , 795.3[M+H] + , 829.4[M+Cl] - .
Embodiment 3
[0165] ent-1α,6β,7β-trihydroxy-(14β-O-(2-formylbenzoic acid-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4)-oxygen Butyl ester))-15-oxo-7,20-oxo-16-kaurene
[0166] With reference to the synthesis method of Example 1, 52 mg of white solid (yield 50.1%) was obtained: mp.108-110° C.; IR (KBr) υ max 3384, 2952, 2025, 1715, 1615, 1553, 1450, 1368, 734, 685cm -1 ; 1 H NMR (CDCl 3 , 300MHz), δ (ppm) 1.10 (6H, s, -CH 3 ), 3.32 (1H, d, J=9.9Hz, 13-CH), 3.50 (1H, m, 1-CH), 3.76 (1H, m, 6-CH), 4.08, 4.34 (each 1H, dd, J A =J B =8.4HZ, 20-CH 2 ), 4.44 (2H, m, -CH 2 ), 4.50 (2H, t, J=5.4Hz, -CH 2 ), 5.30 (1H, s, 1-OH), 5.56 (1H, s, 17-CH 2), 6.04 (1H, d, J=10.5Hz, 6-OH), 6.09 (1H, s, 14-CH), 6.61 (1H, s, 17-CH 2 ), 7.53 (3H, m, Ar-H), 7.61 (2H, m, Ar-H), 7.76 (2H, m, Ar-H), 8.06 (2H, d, J=7.8Hz, Ar-H) ; MS (ESI) m / z: 826.1 [M+NH 4 ] + , 809.0[M+H] + , 843.3[M+Cl] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com