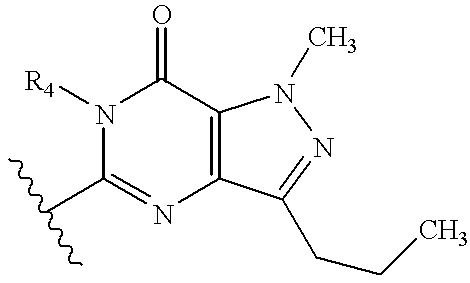
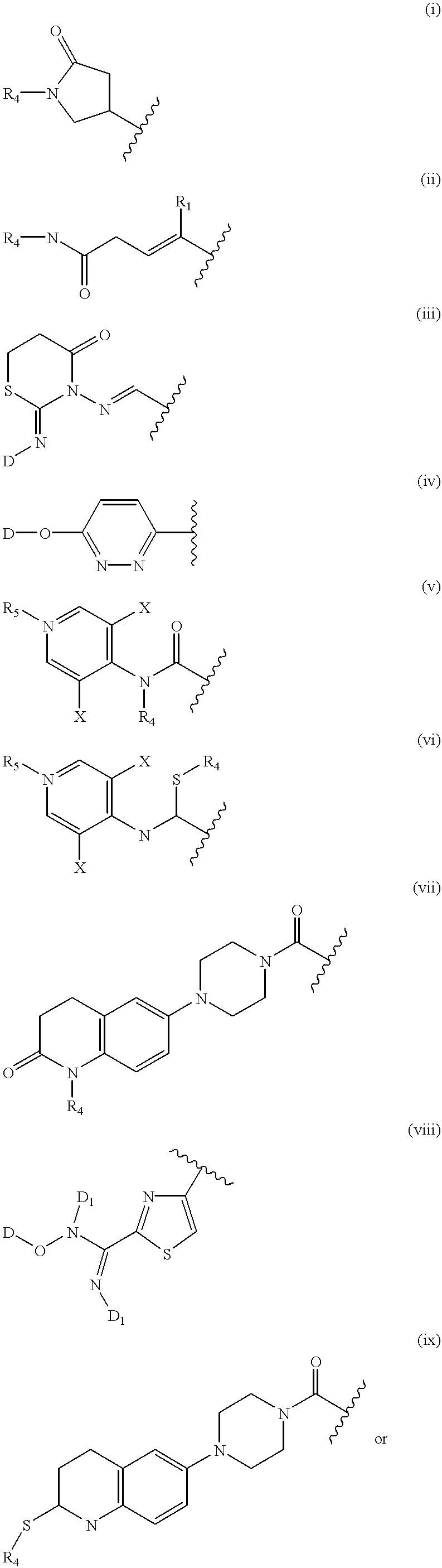
Nitrosated and nitrosylated phosphodiestrase inhibitor compounds, compositions and their uses
a technology of nitrosylated phosphodiestrase inhibitors and compositions, applied in the field of pharmaceuticals, can solve the problems of unexplored effects of modified phosphodiesterase inhibitors directly or indirectly linked with nitric oxide adducts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
The following definitions may be used throughout the specification.
The term "lower alkyl" as used herein refers to branched or straight chain alkyl groups comprising one to ten carbon atoms, including methyl, ethyl, propyl, isopropyl, n-butyl, t-butyl, neopentyl and the like.
The term "alkoxy" as used herein refers to R.sub.50 O-- wherein R.sub.50 is a lower alkyl group as defined herein. "Alkoxy groups" include, for example, methoxy, ethoxy, t-butoxy and the like.
The term "alkoxyalkyl" as used herein refers to an alkoxy group as previously defined appended to an alkyl group as previously defined. Examples of alkoxyalkyl include, but are not limited to, methoxymethyl, methoxyethyl, isopropoxymethyl and the like.
The term "hydroxy" as used herein refers to --OH.
The term "hydroxyalkyl" as used herein refers to a hydroxy group as previously defined appended to a lower alkyl group as previously defined.
The term "alkenyl" as used herein refers to a branched or straight chain C.sub.2 -C.sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| covalent | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com