Pharmaceutical preparations containing myristamidopropyldimethylbenzylammonium chloride
A myristoyl amino, pharmaceutical preparation technology, applied in the directions of medical preparations containing active ingredients, drug combinations, drug delivery, etc., can solve the problems of limited scope of effectiveness, insufficient efficacy, inability to combine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
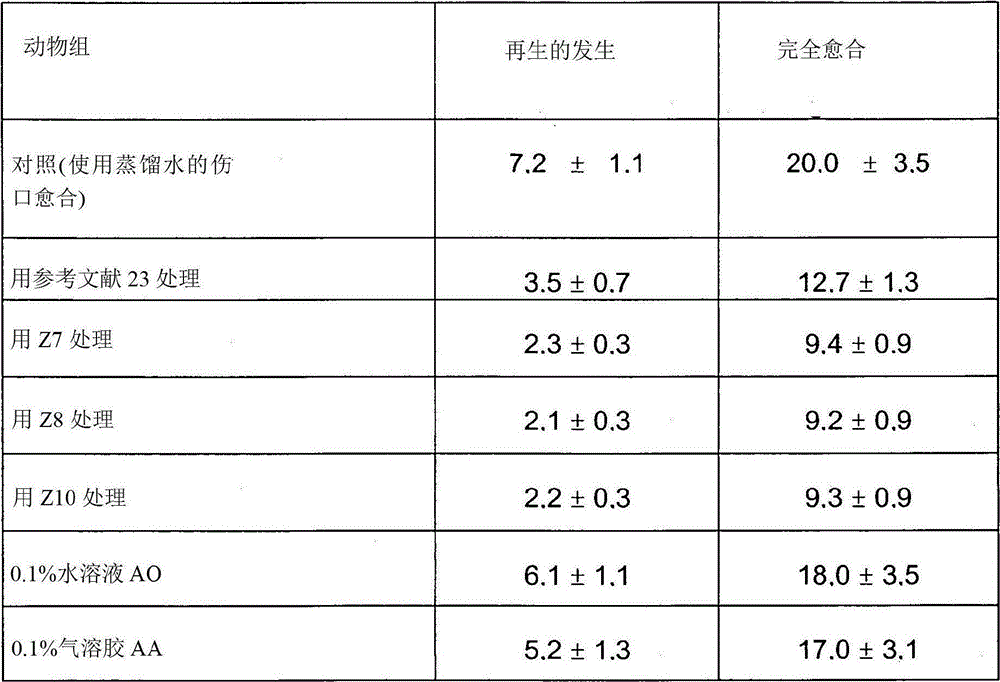
Image
Examples
Embodiment 1
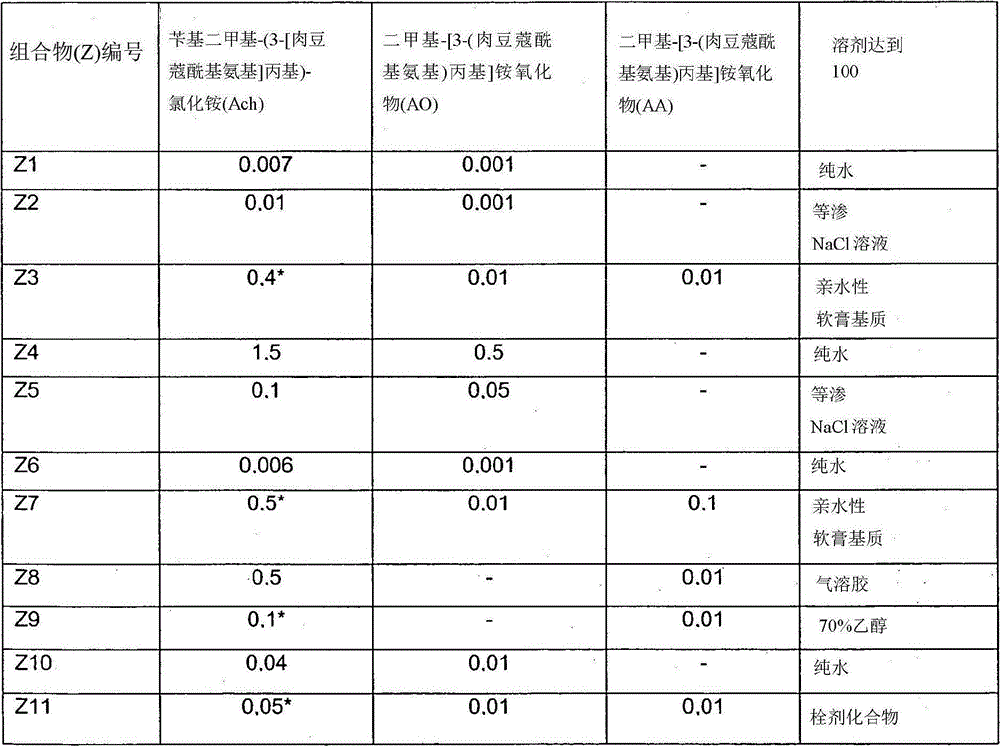
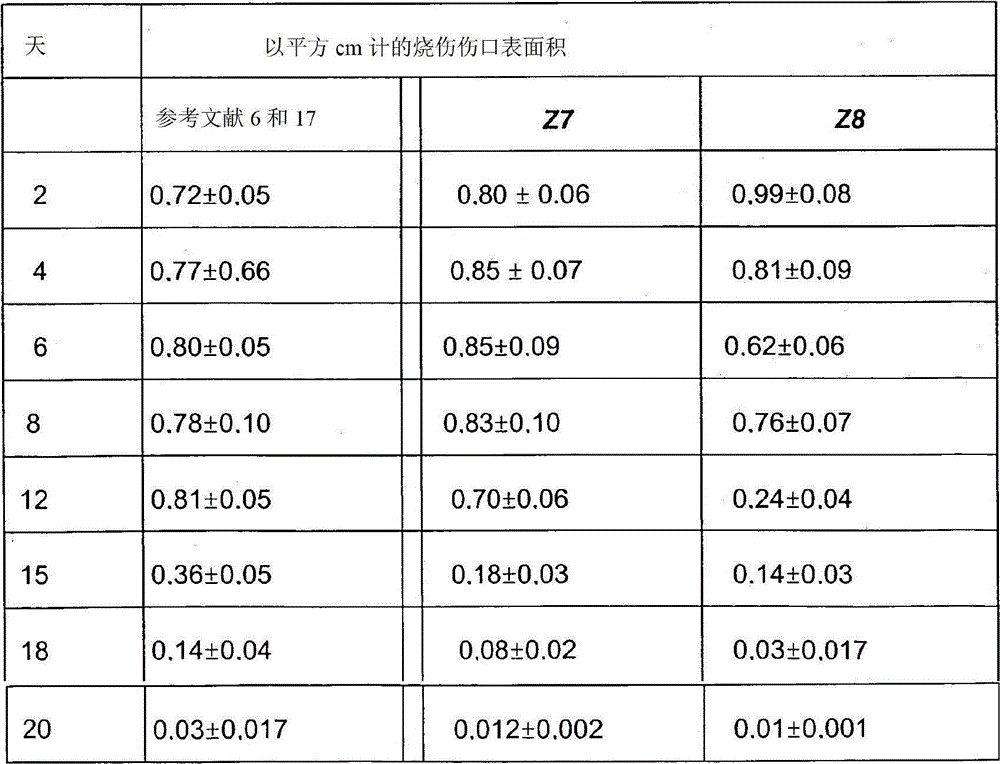
[0034] To demonstrate the technical solution of the invention, various variants of the composition of the formulation of the patent application were used. In Table 1 some of the compositions investigated, in particular those used in the Examples discussed below, are shown.
[0035] Variants of the composition of Table 1 (in % by weight)
[0036]
[0037] Notes: *Monohydrate
Embodiment 2
[0039] The minimum inhibitory concentration (MIC) for a test culture of a coronavirus (human coronavirus OC43) was determined in vitro on human embryonic kidney cell cultures. Using composition Z1 (two components in a ratio of 6:1) and the formulation of reference 3 (pure benzyldimethyl-(3-myristoylamino]propyl) chlorinated ammonium) and pure dimethyl-[3-(myristoylamino)propyl]amine oxide (AA). Research was conducted in parallel through the following protocols:
[0040] Phase 1 test virus + myrismidopropyl dimethyl benzyl ammonium chloride (myramistin) (appropriate concentration)
[0041] Phase 2 incubation (2 hours)
[0042] Phase 3 Mix the neutralizing substance of the composition (eg 25% embryonic serum from cows) and incubate the composition for 10-20 min.
[0043] Stage 4 infection of susceptible cell cultures (e.g. human embryonic kidney)
[0044] Phase 5 incubation (5-8 days)
[0045] Results of Phase 6 assays for hemadsorption and cytopathic effects and symplast for...
Embodiment 3
[0051] Guinea pigs and rabbits were used to study the local irritation caused by the formulations of the patent application to the skin and connective tissue membranes as well as to the vaginal mucosa. Dogs were used to study local irritation of the urethral and bladder mucosa.
[0052] The data found demonstrate that application of compositions Z4 and Z9 to the skin does not cause visible and histological changes over the course of 40 days. Increasing the total concentration of ingredients in the formulation to 5.0% by weight resulted in mild xeroderma and insignificant atrophy of the epidermis upon application to day 30.
[0053] Meanwhile, in references 8, 20, and 23, solutions of pure Ach already caused skin irritation at concentrations exceeding 1% by weight, and higher concentrations (2-3% by weight) brought Come visibly greater skin changes.
[0054] Skin irritation was evident by day 10 when a 0.01% solution of pure AA in 70% alcohol was applied.
[0055] A solution...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap