Immunoassay for chromogranin A, antibodies and kit
A kit and a technology for detecting antibodies, applied in the direction of anti-animal/human immunoglobulin, biological testing, immunoglobulin, etc., can solve problems such as assay methods, false negatives, inferior polyclonal antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Monoclonal antibodies
[0058] has basically passed and Milstein's method in 1975 prepared monoclonal antibodies (also expressed as antibody 2E8) that specifically reacted with the polypeptides represented by the 236th to 251st amino acid sequences of the human CGA amino acid sequence, and with the 264th to 279th A monoclonal antibody (also denoted herein as antibody 1H6) specifically reacts with the polypeptide indicated by the amino acid sequence.
[0059] Antibody 2E8 was used as a capture antibody in the Elisa; antibody 1H6 was used as a detection antibody bound to HRP.
Embodiment 2
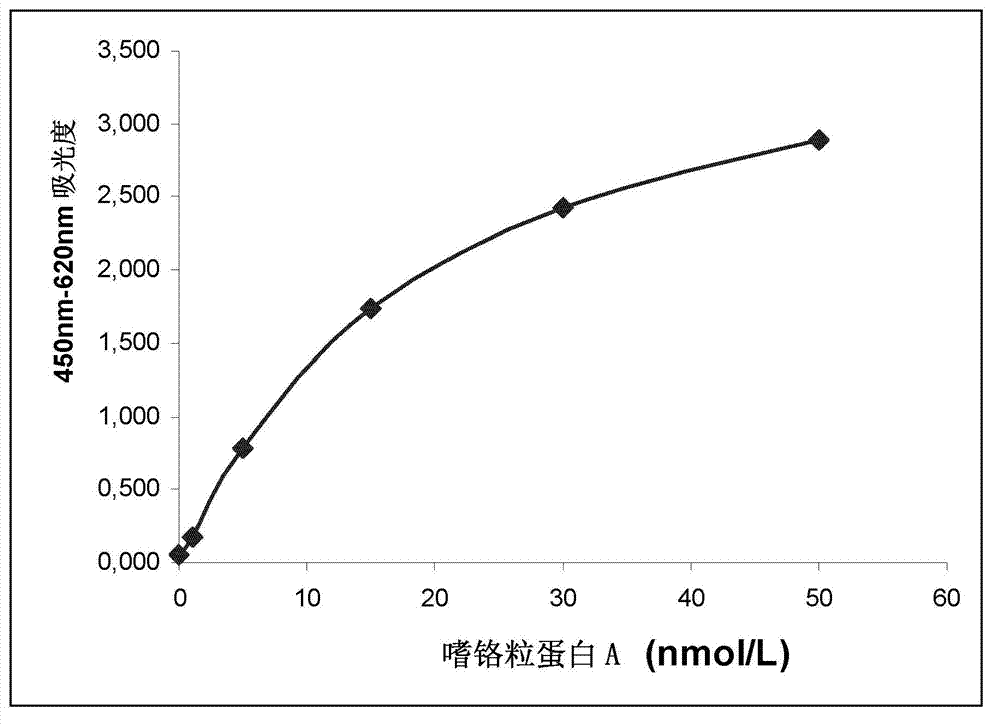
[0061] Calibration curve of CGA ELISA
[0062] A set of 6 calibrators are provided, the values of which are calibrator 1: 0nmol / L, calibrator 2: 1nmol / L, calibrator 3: 5nmol / L, calibrator 4: 15nmol / L, calibrator 5: 30nmol / L, calibrator 6: 50nmol / L.
[0063] The calibrator in the kit is a synthetic peptide corresponding to CGA. Peptide calibrators set up to produce responses equivalent to purified native CGA fragments (Stridsberg, M et al 1993, Stridsberg et al 1995)
[0064] The OD of the six calibrators was plotted against the nmol / L value to construct a calibration curve.
[0065] The results of calibrator measurements are in Table 1 and figure 1 described in.
[0066] Table 1
[0067]
Embodiment 3
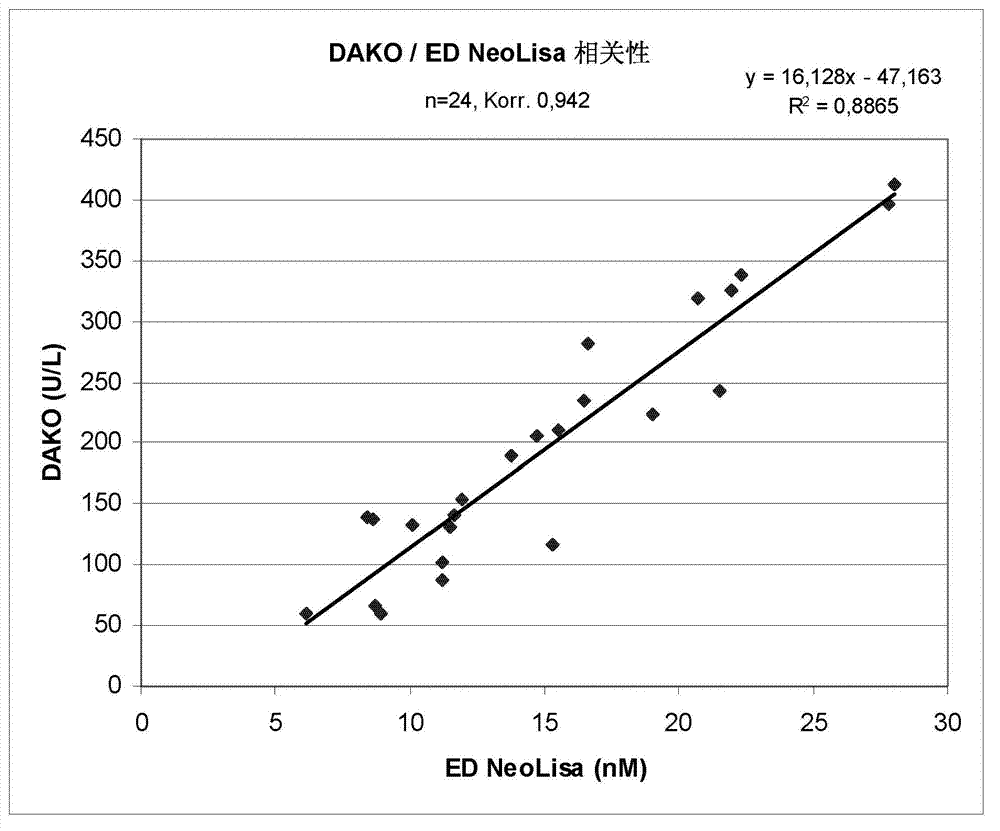
[0069] Correlation between the test system of embodiment 1 and commercial test kit (Dako)
[0070] For this comparison, the example of the present invention was used alongside the assay kit of the Chromogranin A ELISA kit (code K0025) commercially available from Dako (Dako Denmark A / S; Produktionsvej 42; DK-2600 Glostrup; Denmark). The testing system of 1 tests a batch of patient samples. The commercially available assay kit is widely used and is based on a simplified double polyclonal antibody sandwich assay in which the sample is simultaneously treated with a peroxidase-conjugated anti-chromogranin A antibody on a surface coated with anti-chromogranin Antibody A was incubated in the microwells. All antibodies in this test kit are reported to be rabbit polyclonal antibodies. Use the Dako assay kit according to the manufacturer's instructions. In the assay, the OD is read at 450nm and 650nm.
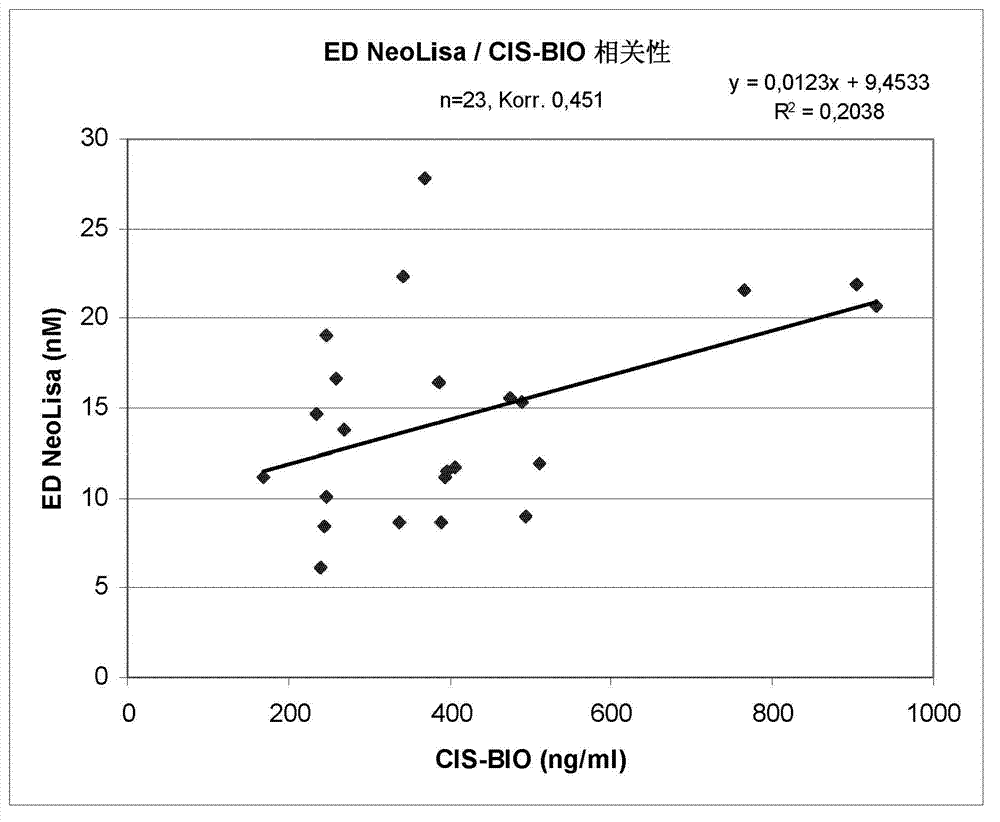
[0071] The results of this comparison are shown in the figure 2 middle. Her...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com