Methods for treating breast cancer
A breast cancer, hormone therapy technology, applied in chemical instruments and methods, pharmaceutical formulations, antibody medical ingredients, etc., can solve the problems of fewer treatment options for patients and a large risk of death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
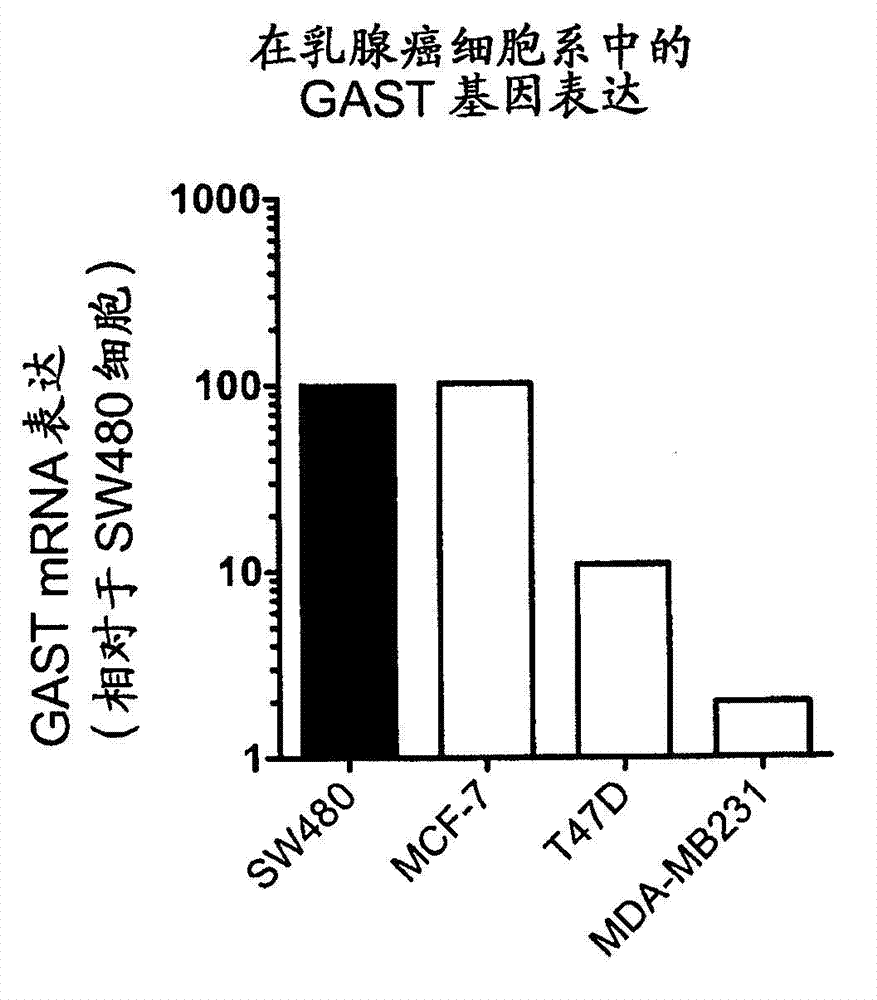
[0287] Example 1 Expression of gastrin gene in metastatic breast cancer cell lines
[0288] This example describes the expression of the gastrin (GAST) gene in metastatic breast cancer cell lines.
[0289] A. Method
[0290] GAST mRNA expression was quantified from RNA preparations of MCF-7, MDA-MB-231 and T47D cell lines using quantitative RT-PCR. Data are presented in comparison to the mRNA expression levels present in the SW480 cell line, a primary colorectal cancer cell line expressing high levels of gastrin mRNA.
[0291] MCF-7 cells are a metastatic human breast cancer cell line. They were originally derived from the pleural effusion of patients diagnosed with breast cancer. MDA-MB-231 cells are metastatic human breast cancer cell lines. They were originally derived from the pleural effusion of patients diagnosed with breast cancer. T47D cells are a metastatic human breast cancer cell line. They were originally derived from the pleural effusion of a patient diagnos...
Embodiment 2
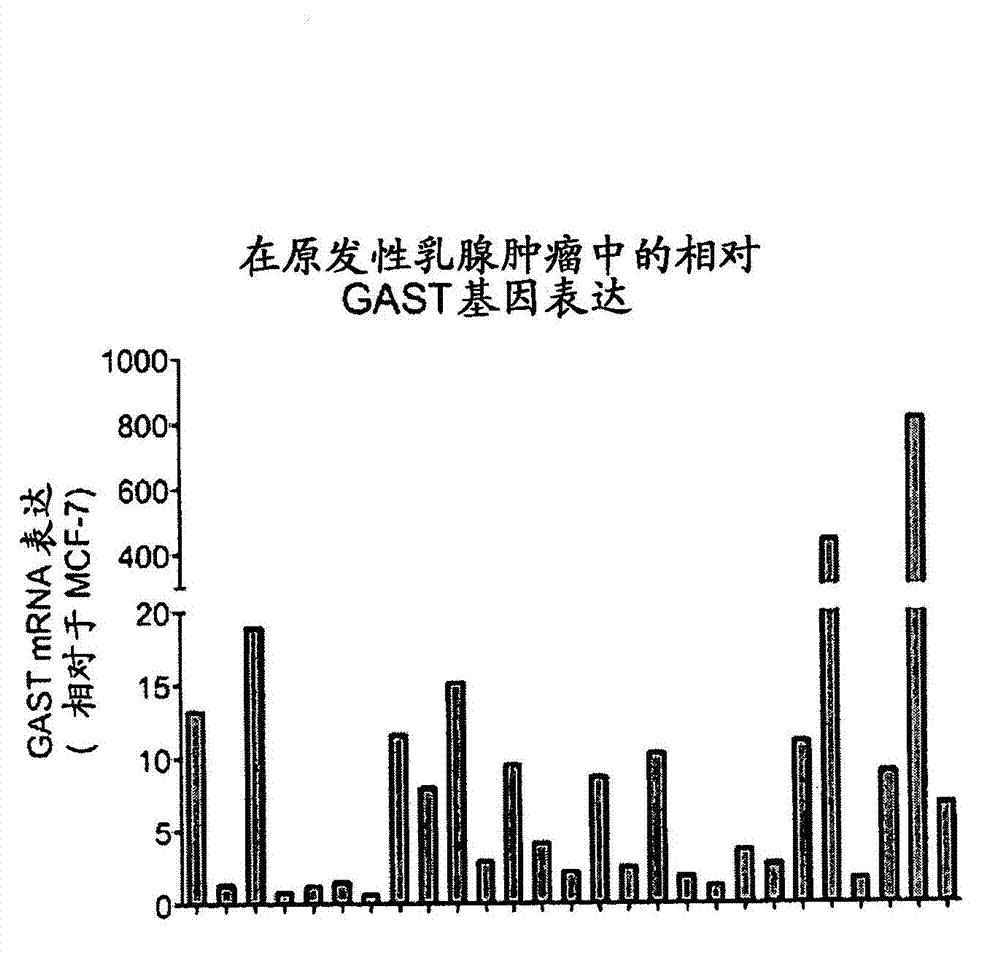
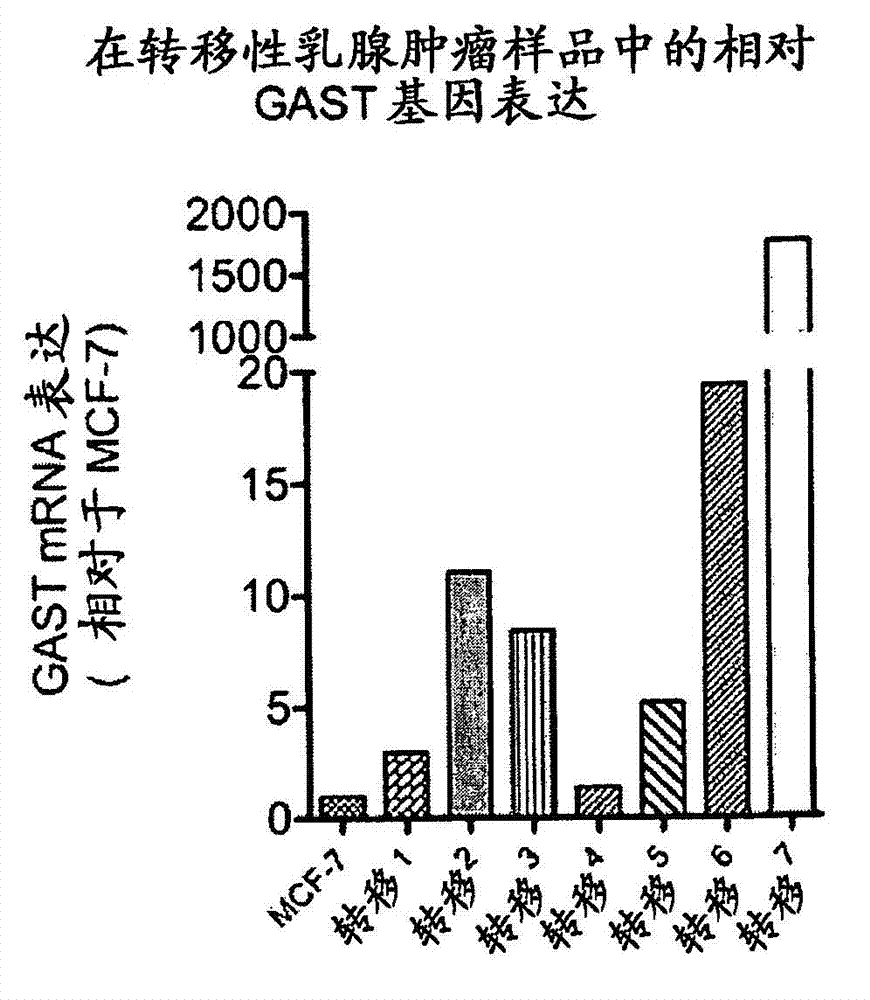
[0294] Example 2 Expression of gastrin gene in primary and metastatic breast tumors surgically removed from patients
[0295] This example describes the expression of the gastrin gene (GAST) in primary and metastatic breast cancer surgically removed from patients.
[0296] A. Method
[0297] Primary and metastatic breast cancers were surgically removed from different patients in accordance with applicable ethical guidelines. RNA was prepared from cancer samples and gastrin mRNA was measured by quantitative RT-PCR. Gastrin mRNA expression in clinical samples was normalized to expression levels in MCF-7 cells.
[0298] B. Results
[0299] A total of 105 primary breast cancers and 25 metastatic breast cancers were tested for gastrin gene expression. Progastrin mRNA in 27 tested primary tumors ( figure 2 ) neutralized in 7 metastatic tumors tested ( image 3 ) can be detected. Thus, gastrin genes are expressed in subtypes of primary and metastatic breast cancers, and expre...
Embodiment 3
[0300] Example 3 Plasma progastrin concentration in breast cancer patients
[0301] This example describes the quantification of plasma levels of progastrin in patients with primary and metastatic breast cancer.
[0302] A. Method
[0303] Plasma progastrin concentrations were measured in healthy individuals as controls, and in 42 patients with metastatic breast cancer who had surgically removed the primary tumor and in 3 patients with primary breast cancer. A total of 104 healthy control samples were obtained from blood banks.
[0304] Plasma or serum progastrin level quantification was performed using a progastrin-specific sandwich ELISA technique similar to one previously described below.
[0305] Wells of a Nunc MaxiSORP 96-well plate were coated with a primary antibody specific for progastrin as follows. Anti-progastrin polyclonal antibodies specific for the carboxy-terminal region of progastrin were diluted to a concentration of 3 μg / ml in a solution of 50 mM, pH 9.6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com