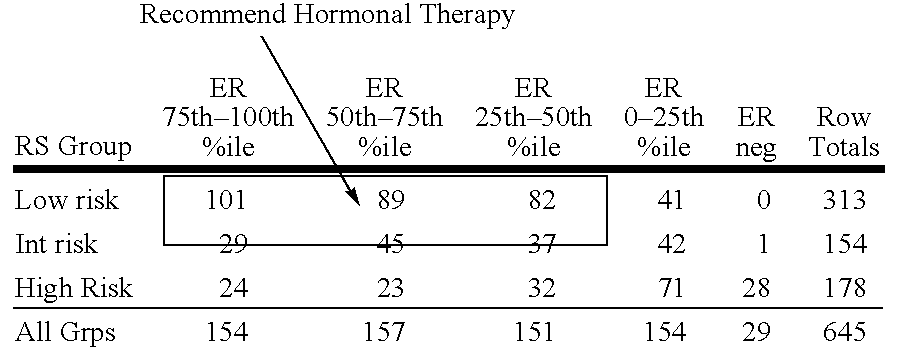
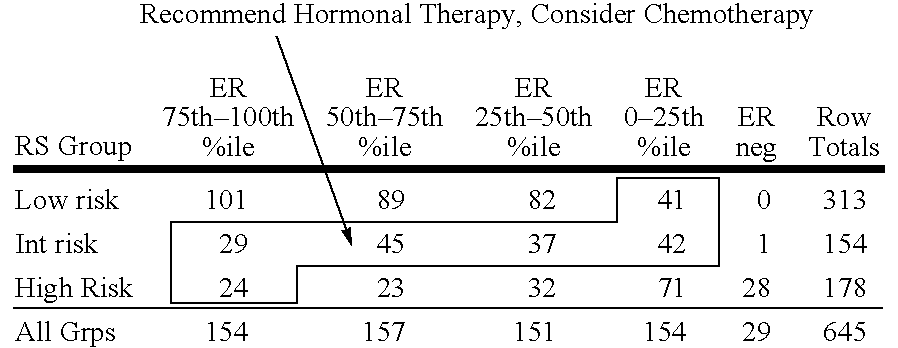
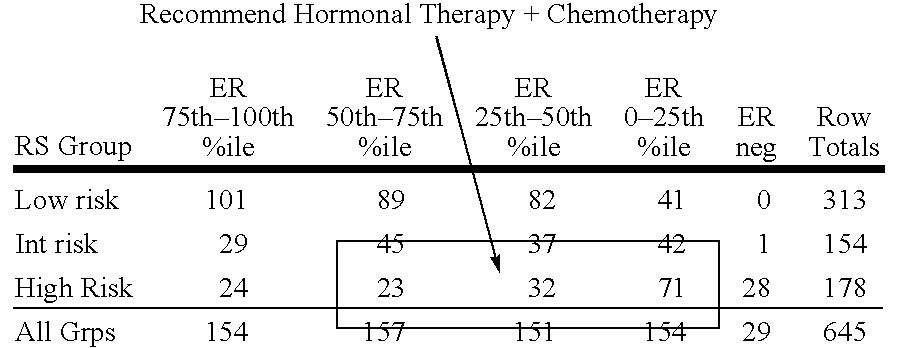
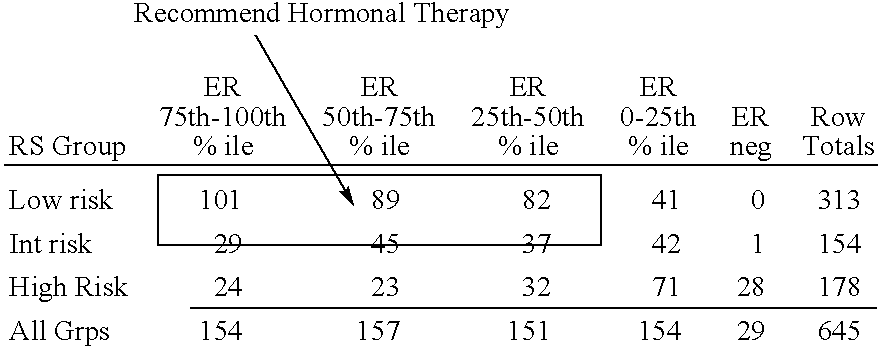
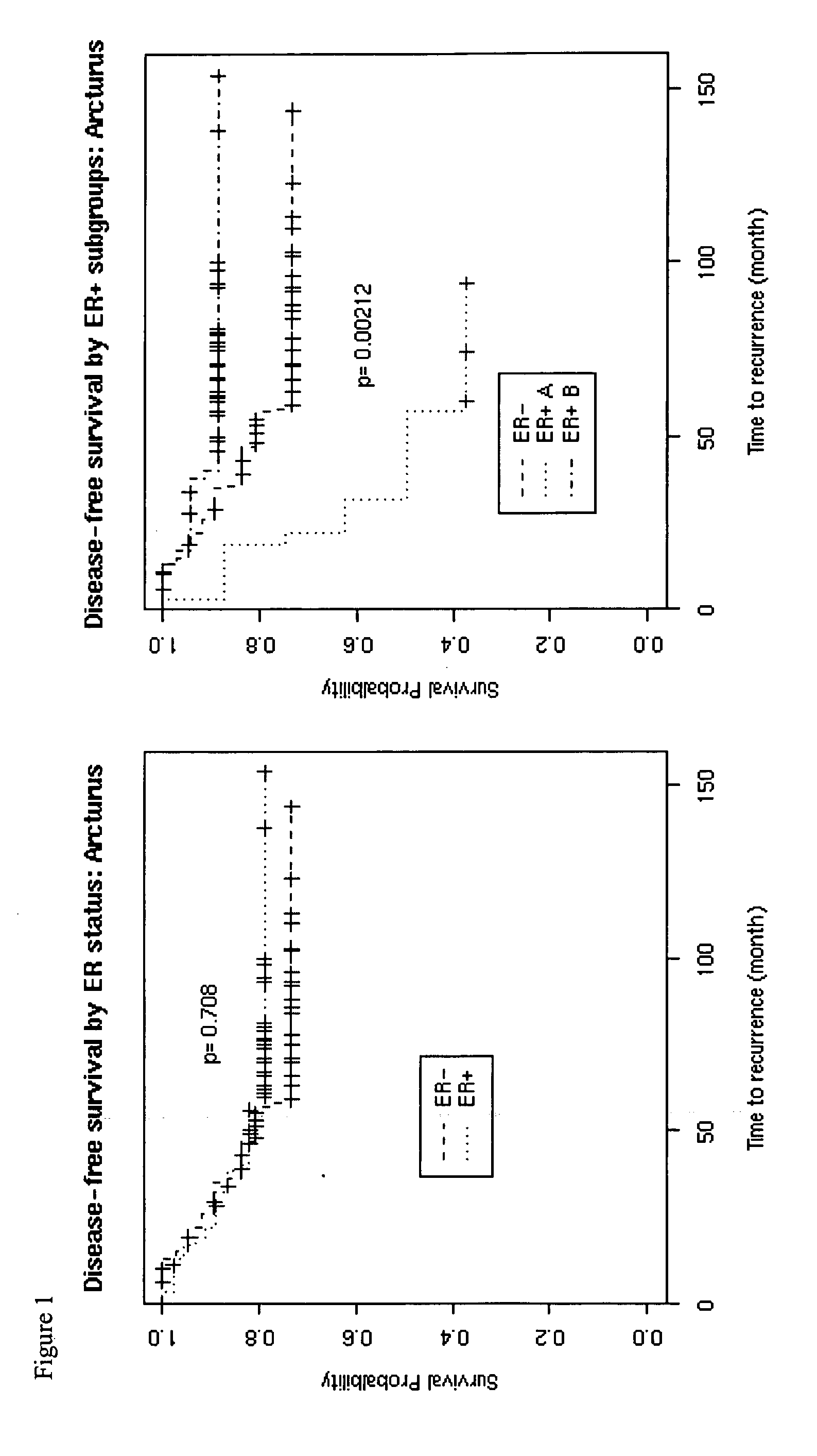
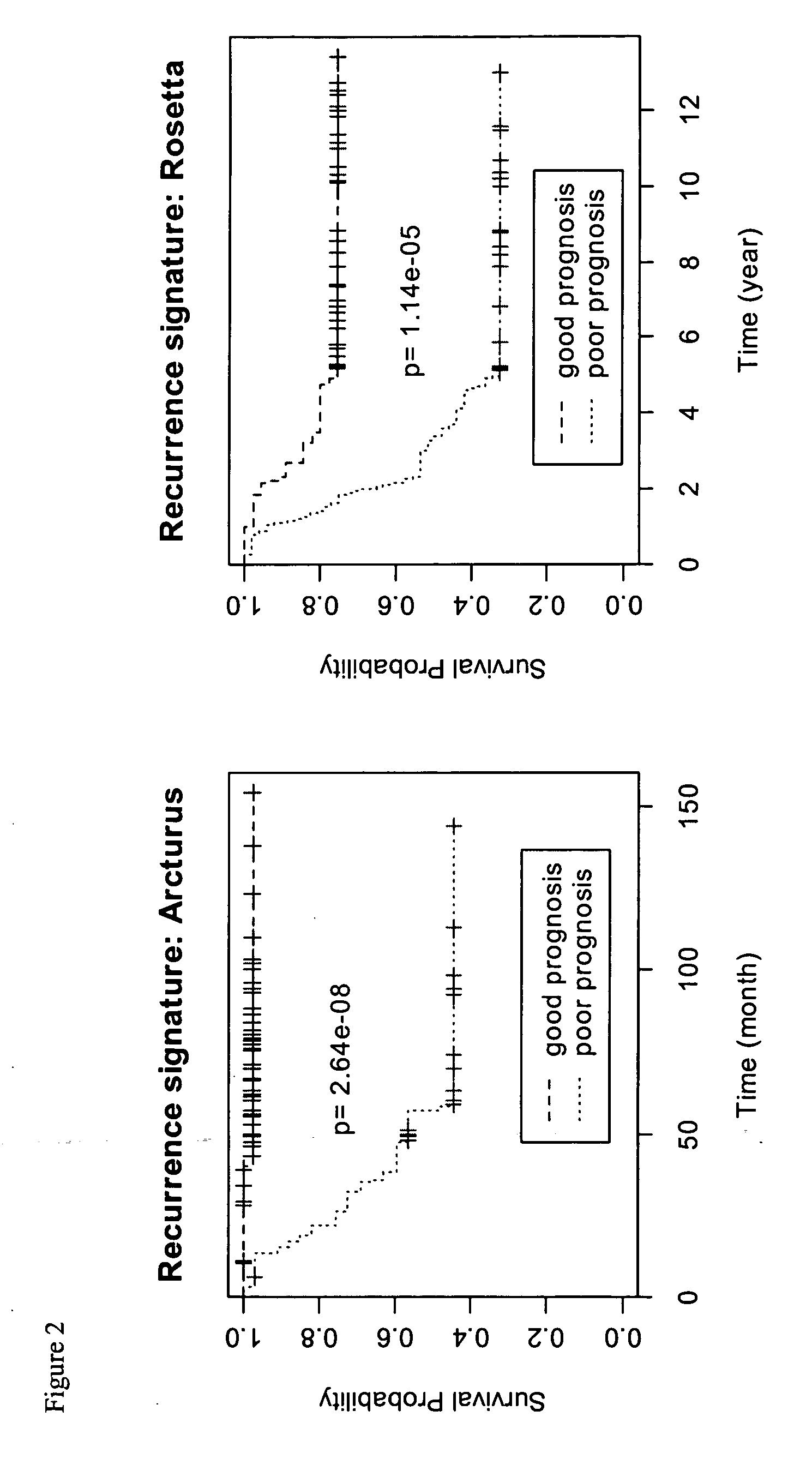
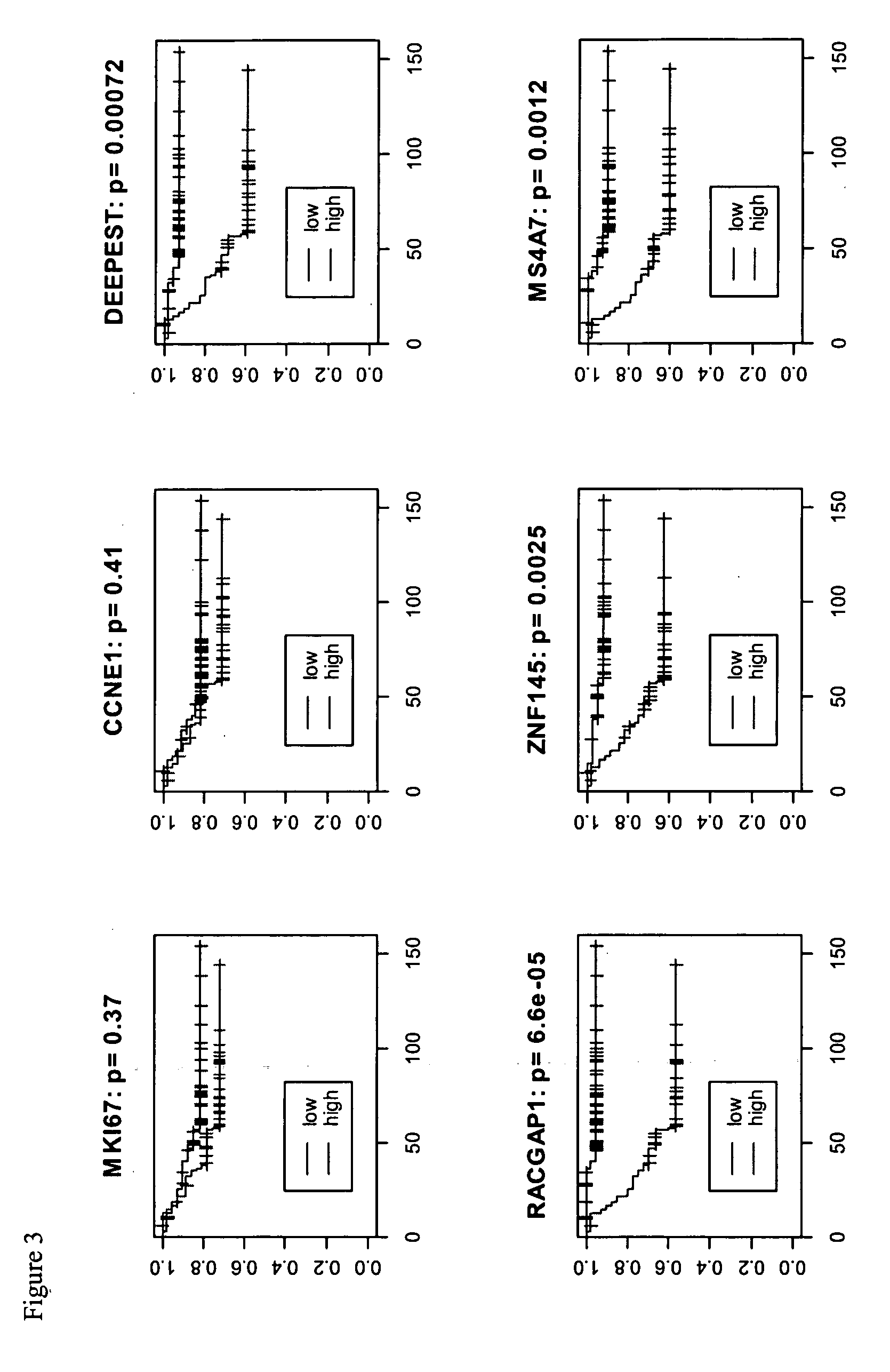
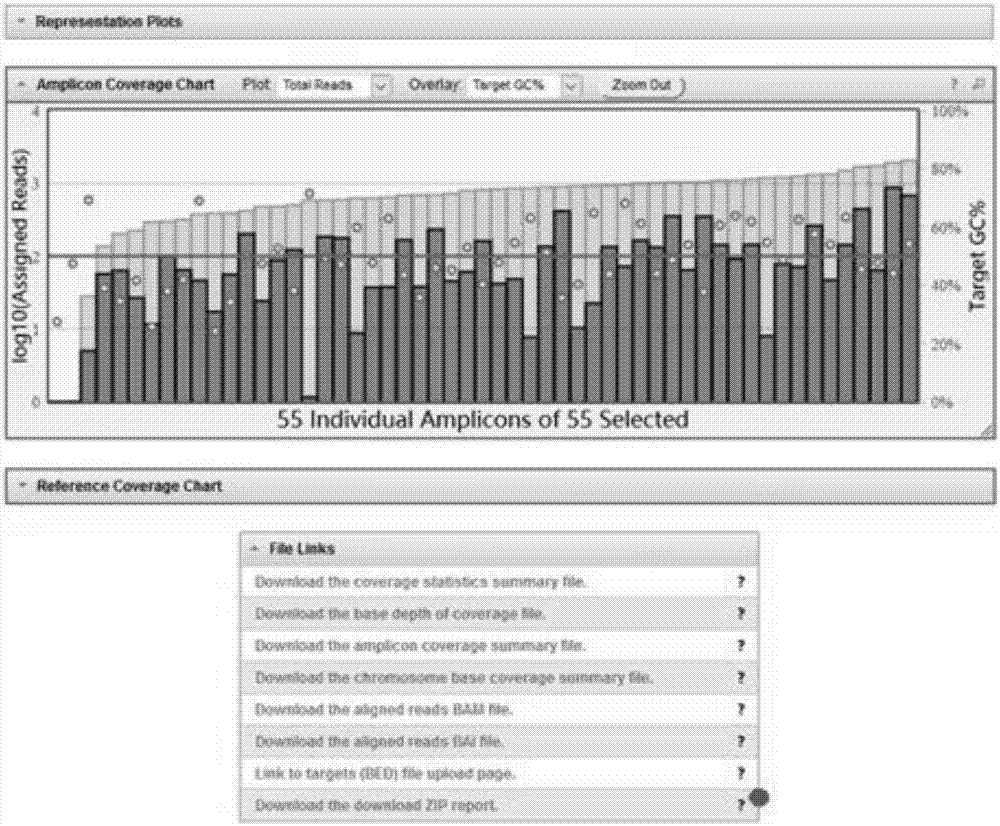
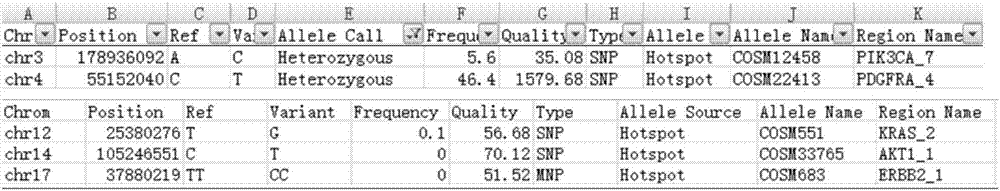
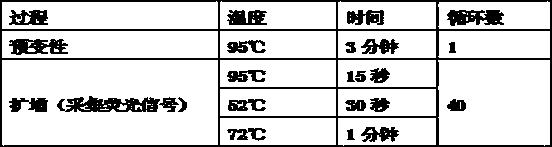
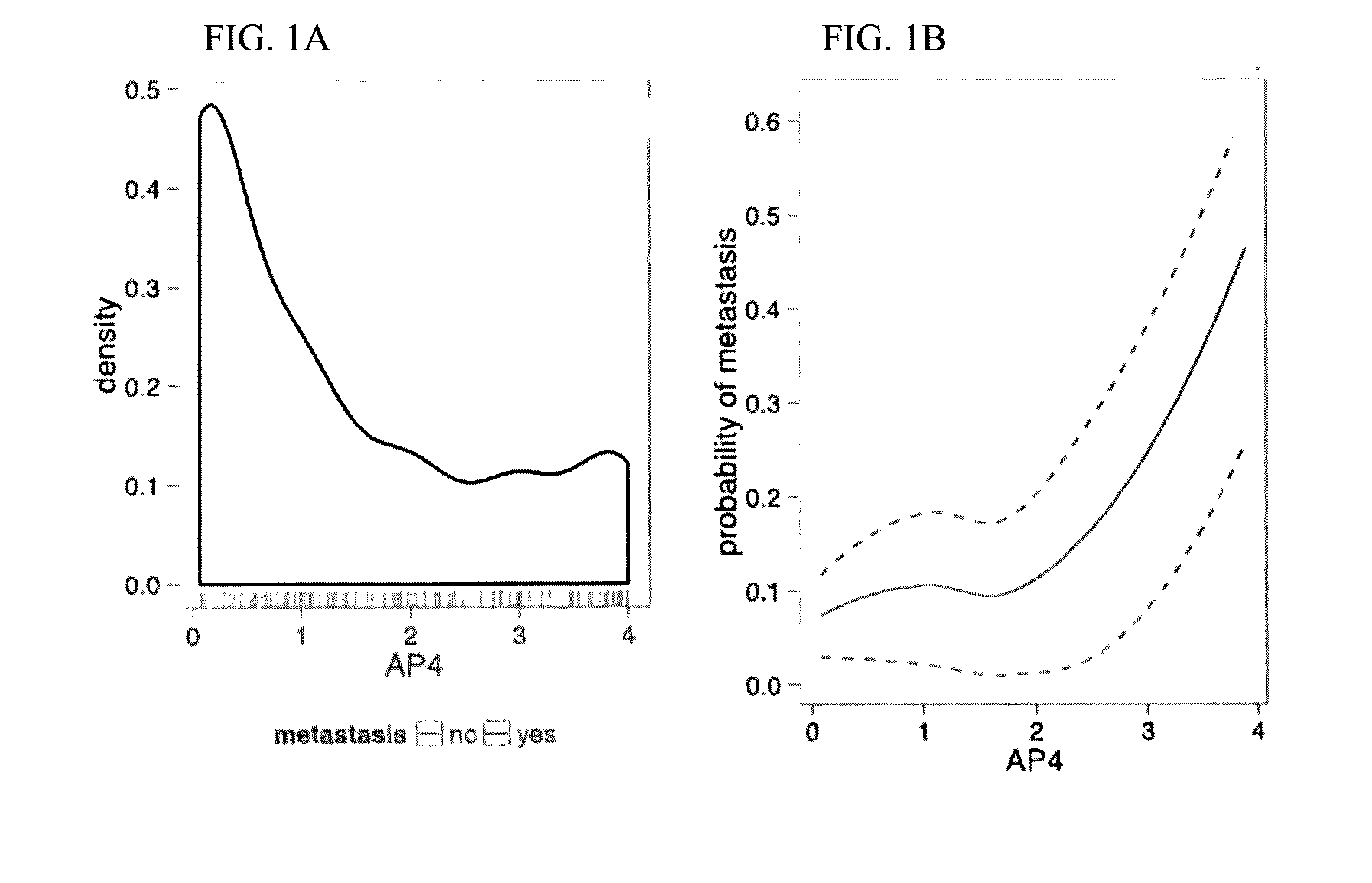
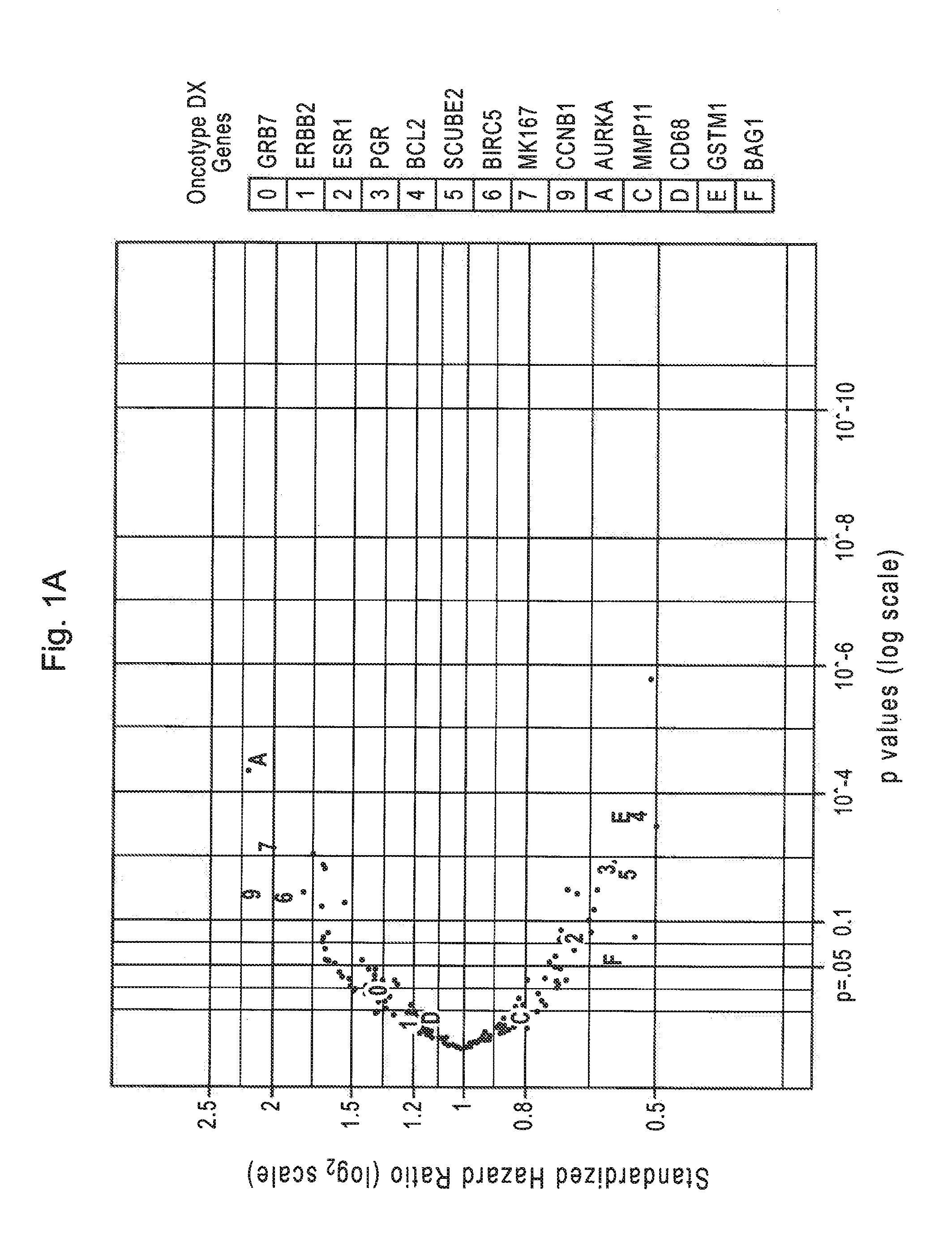
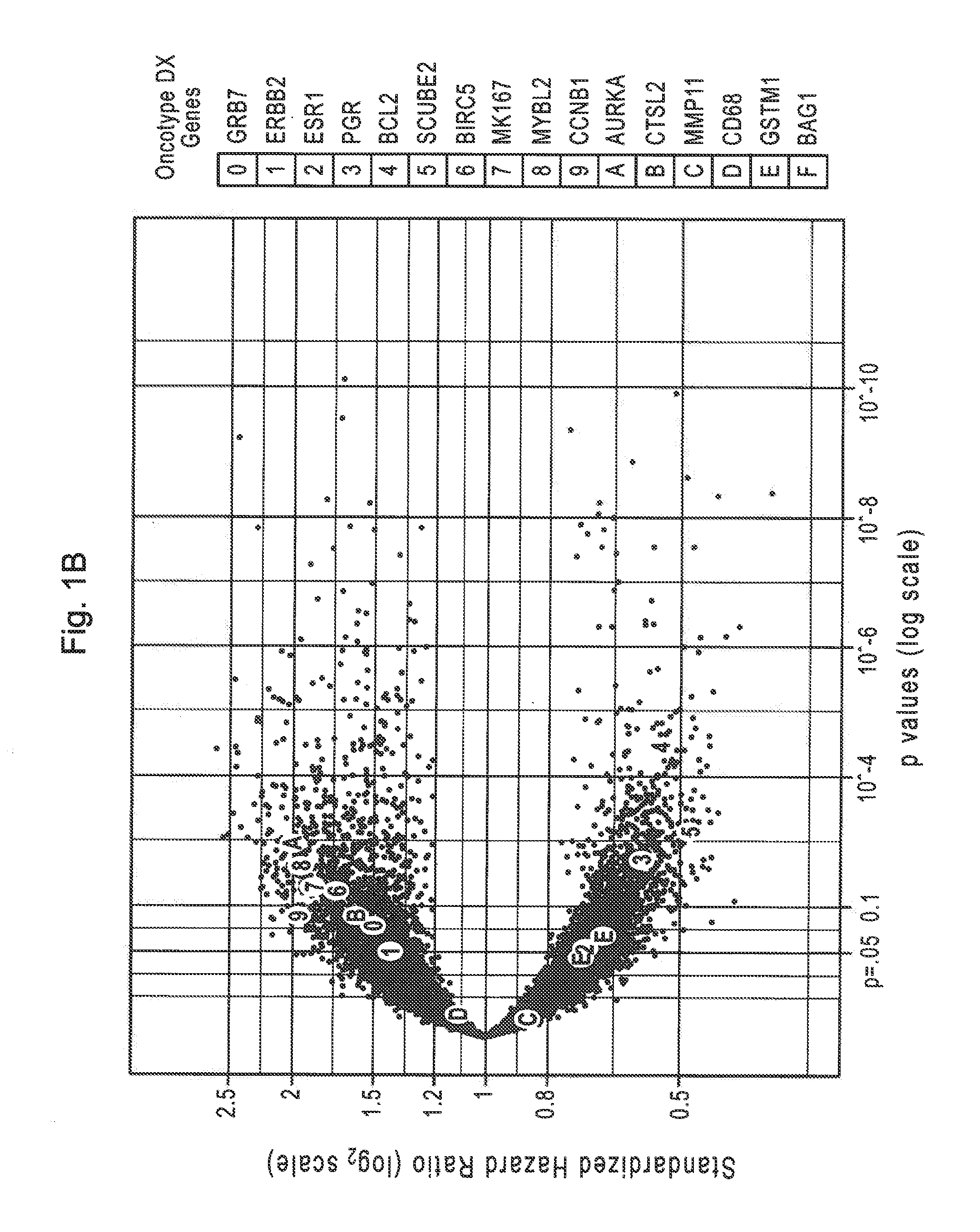
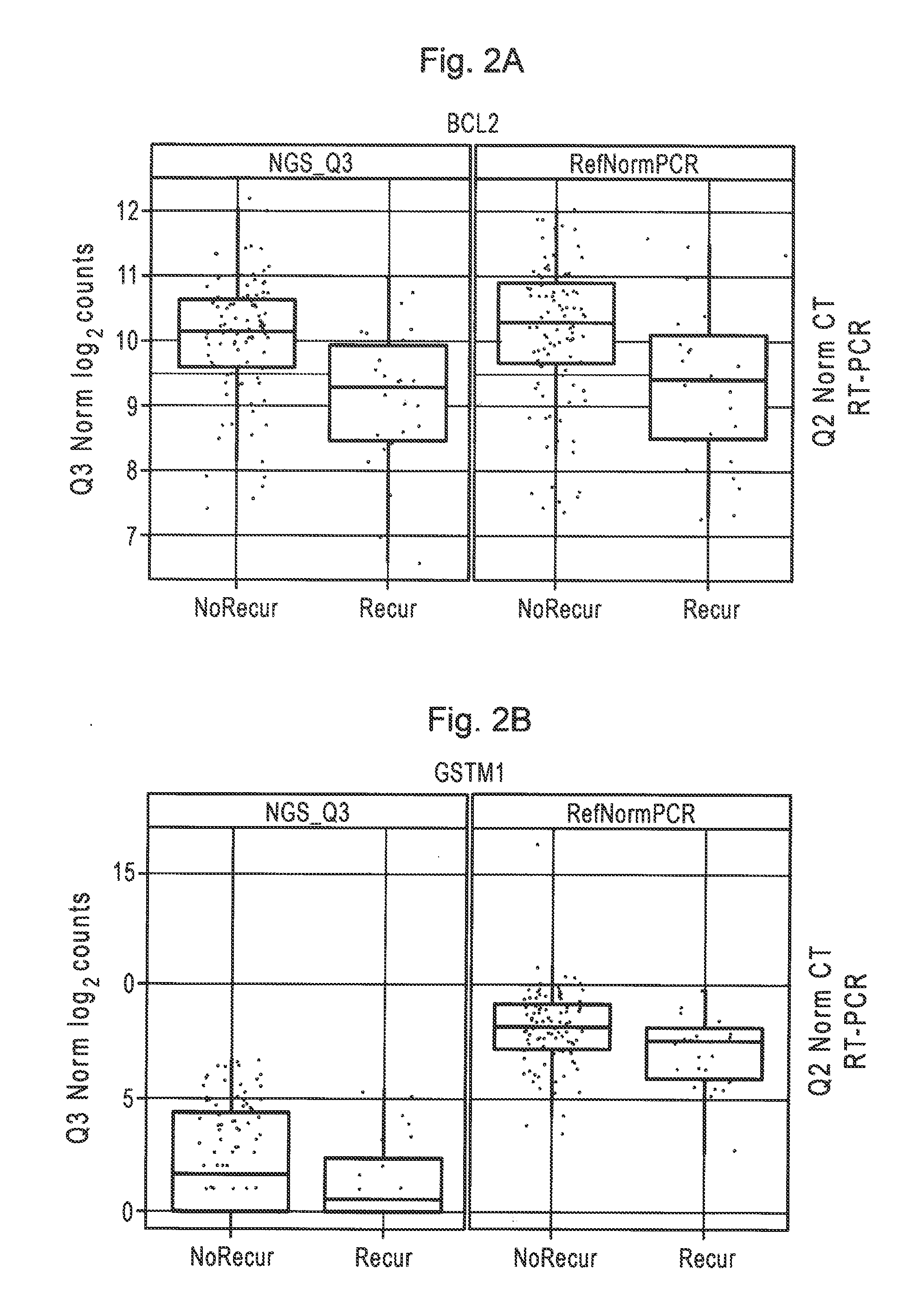
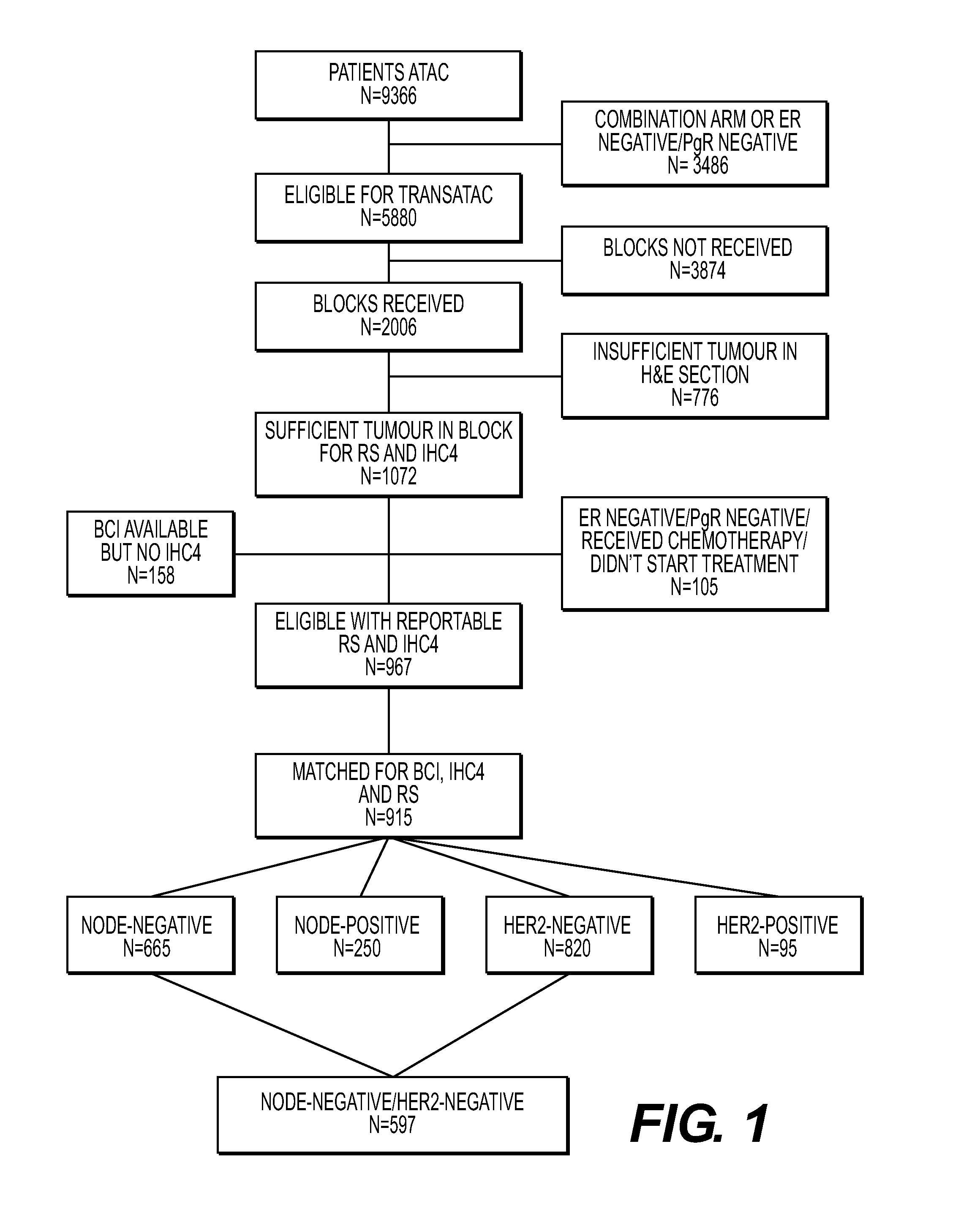
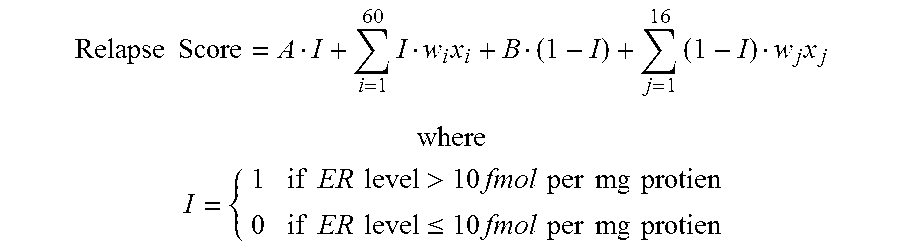Patents
Literature
69 results about "Breast cancer recurrence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Predicting bone relapse of breast cancer
InactiveUS20070031873A1Microbiological testing/measurementBiostatisticsOncologyGene expression profiling
A method of providing predicting relapse of breast cancer in bone is conducted by analyzing the expression of a group of genes. Gene expression profiles in a variety of medium such as microarrays are included as are kits that contain them.
Owner:VERIDEX LCC +1
Methods and systems for assessing risk of breast cancer recurrence
ActiveUS20170091937A1Reduce riskLow experience requirementImage enhancementImage analysisLearning basedLower risk
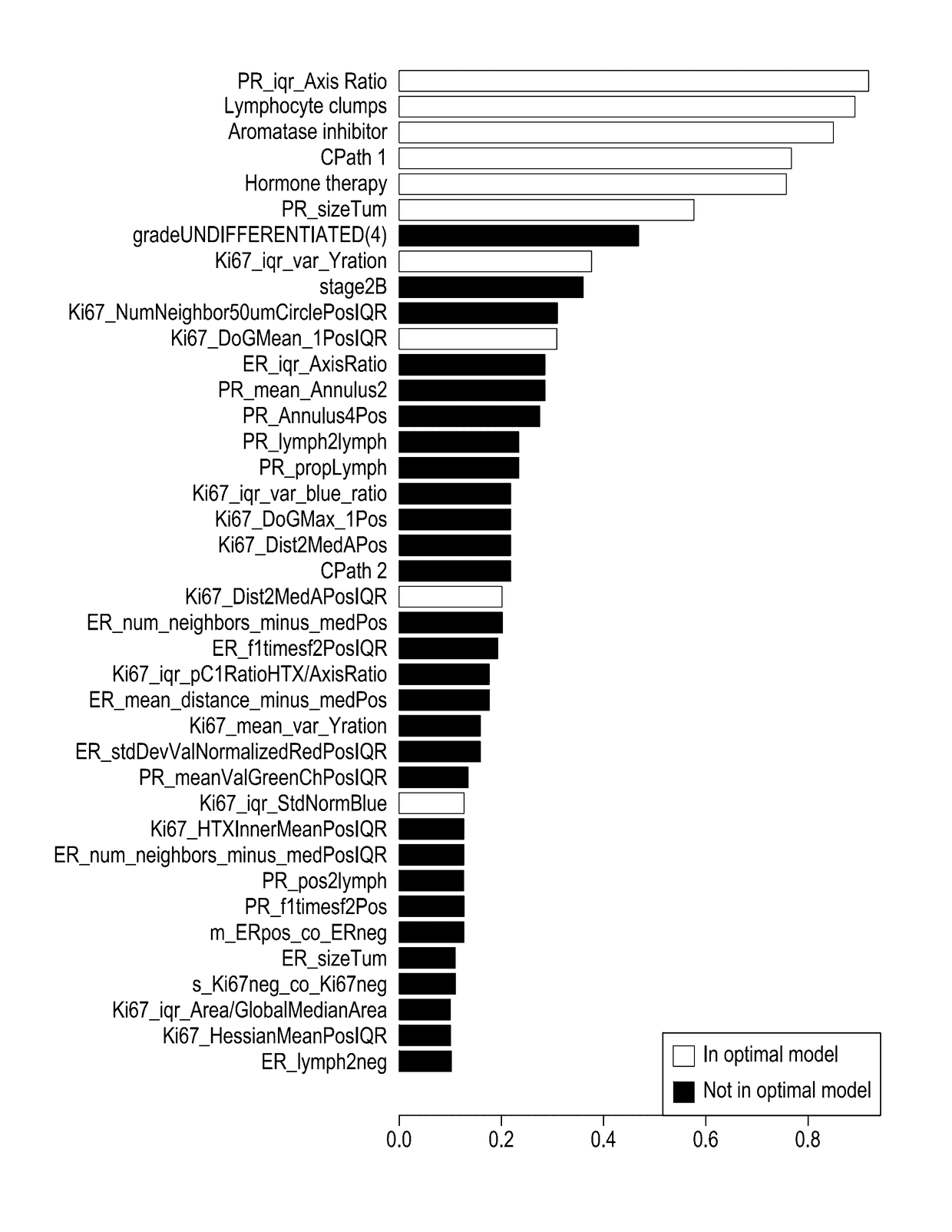
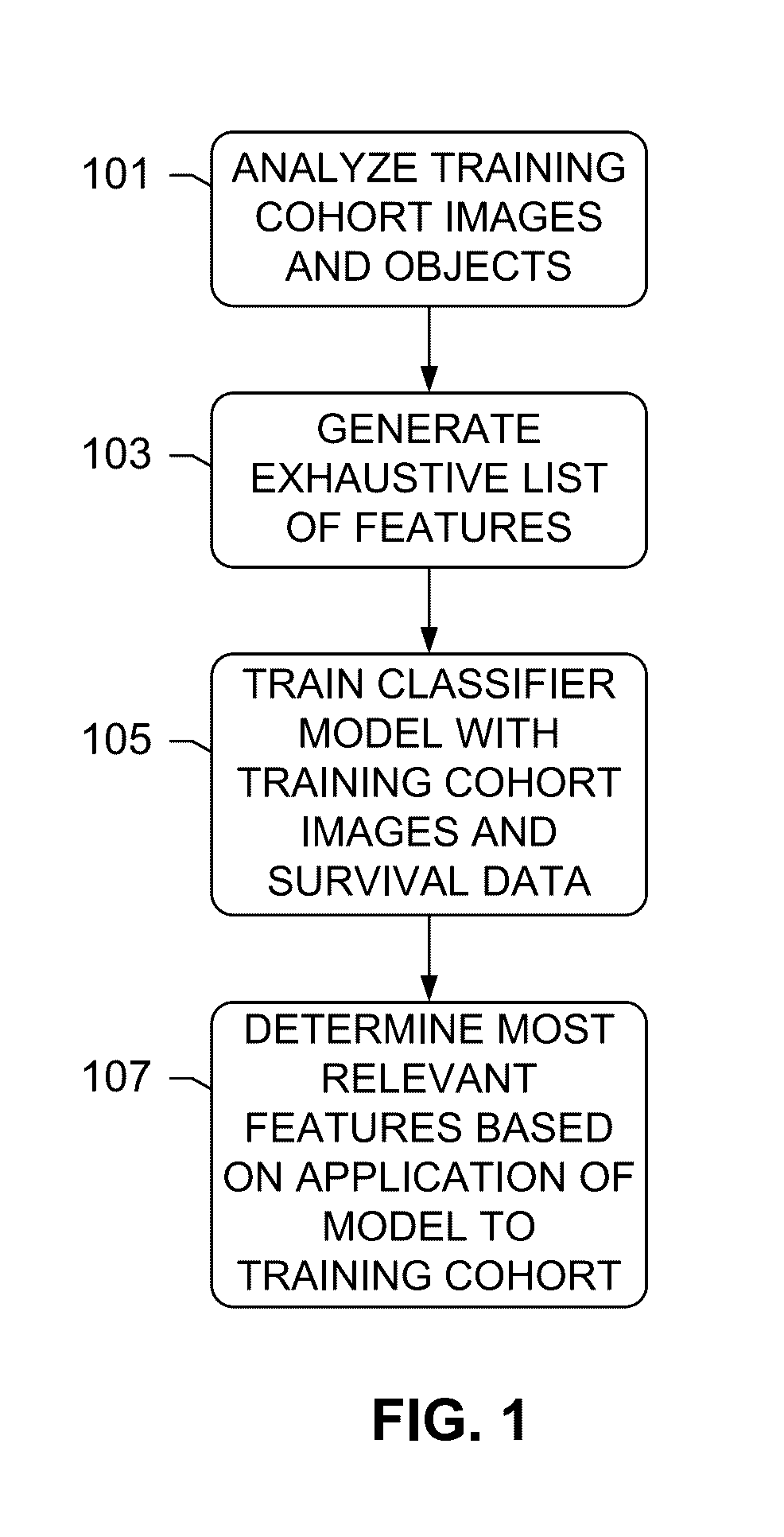
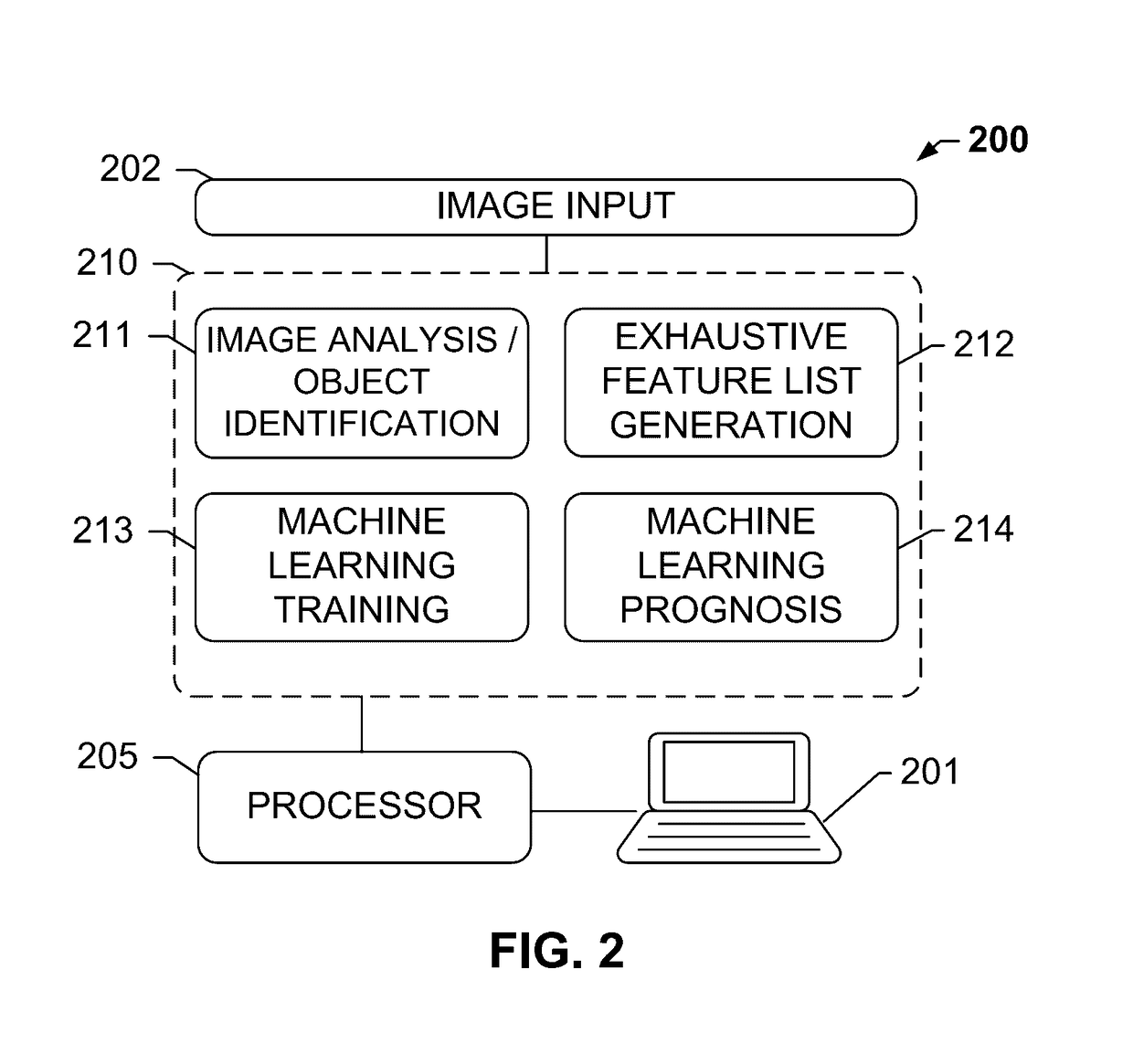
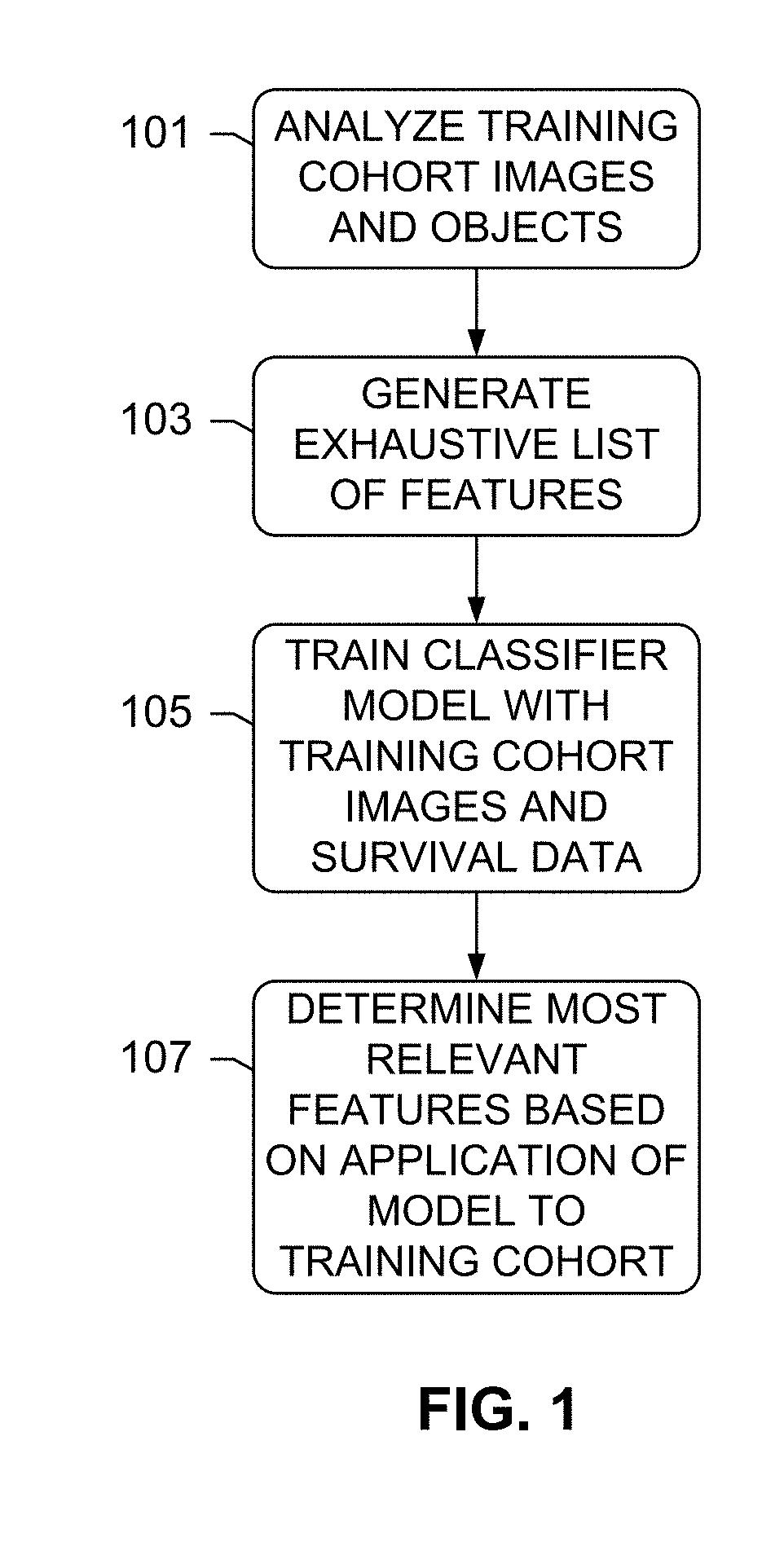
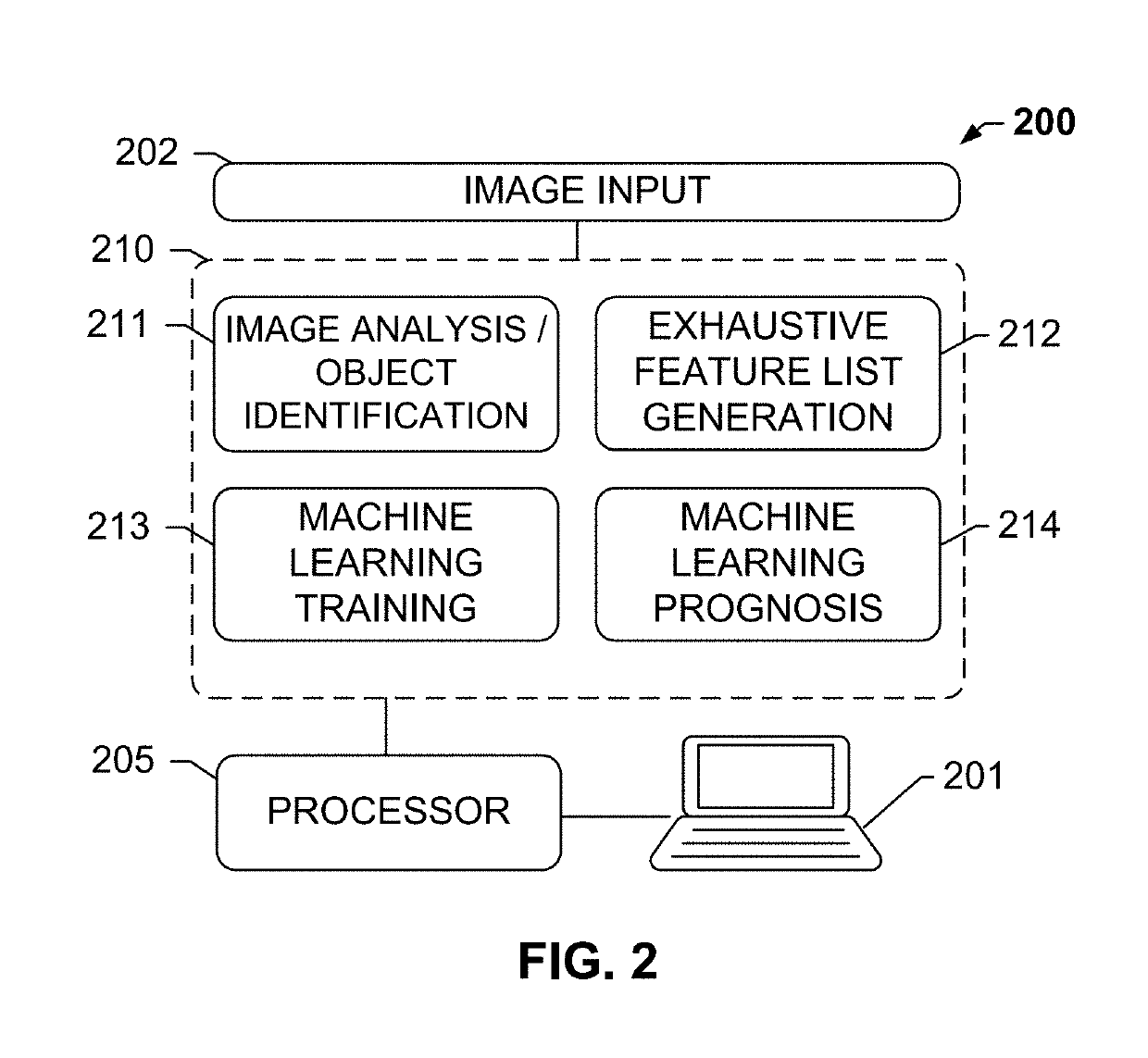
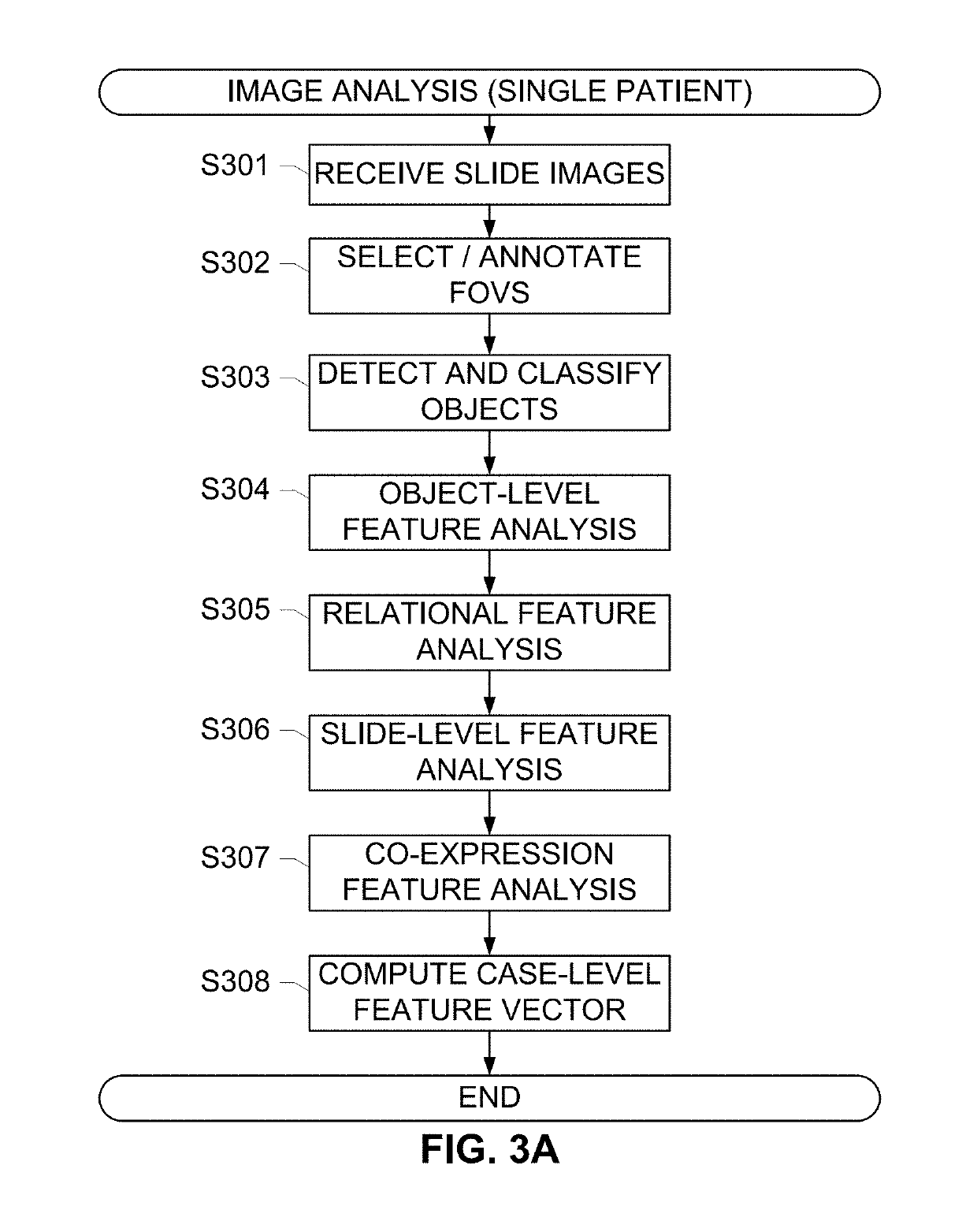
The subject disclosure presents systems and computer-implemented methods for assessing a risk of cancer recurrence in a patient based on a holistic integration of large amounts of prognostic information for said patient into a single comparative prognostic dataset. A risk classification system may be trained using the large amounts of information from a cohort of training slides from several patients, along with survival data for said patients. For example, a machine-learning-based binary classifier in the risk classification system may be trained using a set of granular image features computed from a plurality of slides corresponding to several cancer patients whose survival information is known and input into the system. The trained classifier may be used to classify image features from one or more test patients into a low-risk or high-risk group.
Owner:VENTANA MEDICAL SYST INC +1
Molecular indicators of breast cancer prognosis and prediction of treatment response
ActiveUS20060166231A1Decrease riskReduce riskMicrobiological testing/measurementProteomicsTreatment responseClinical decision
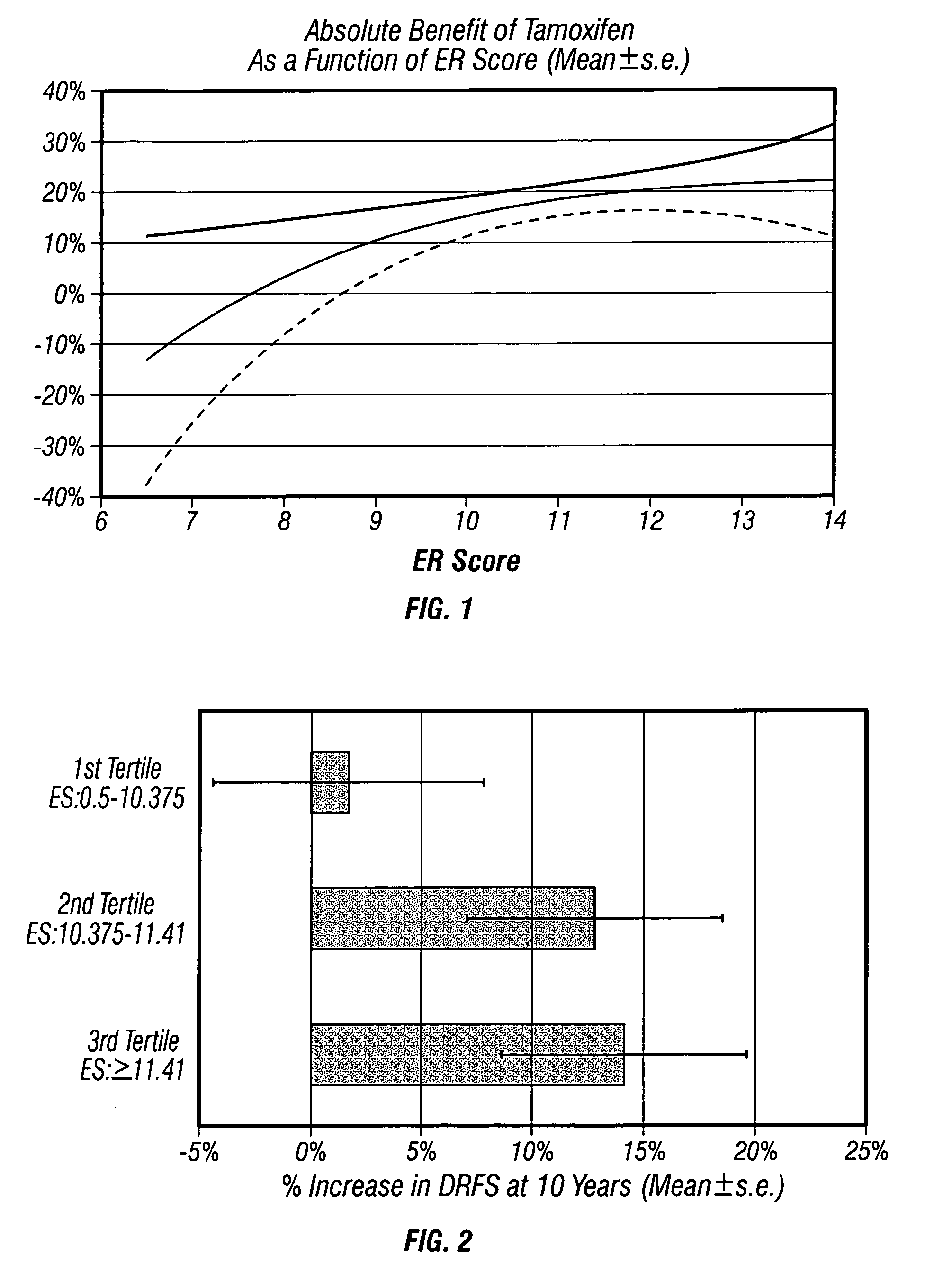
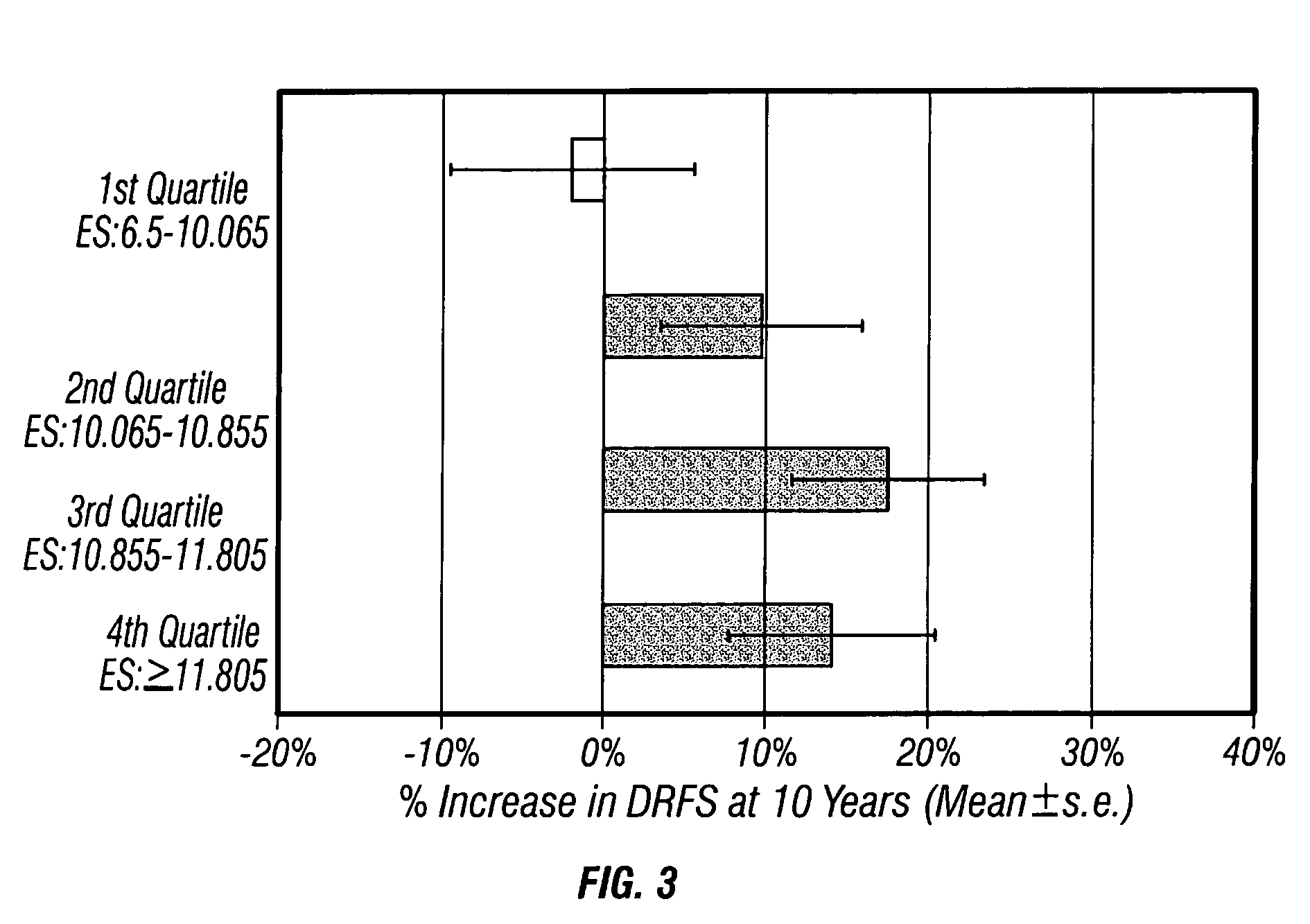
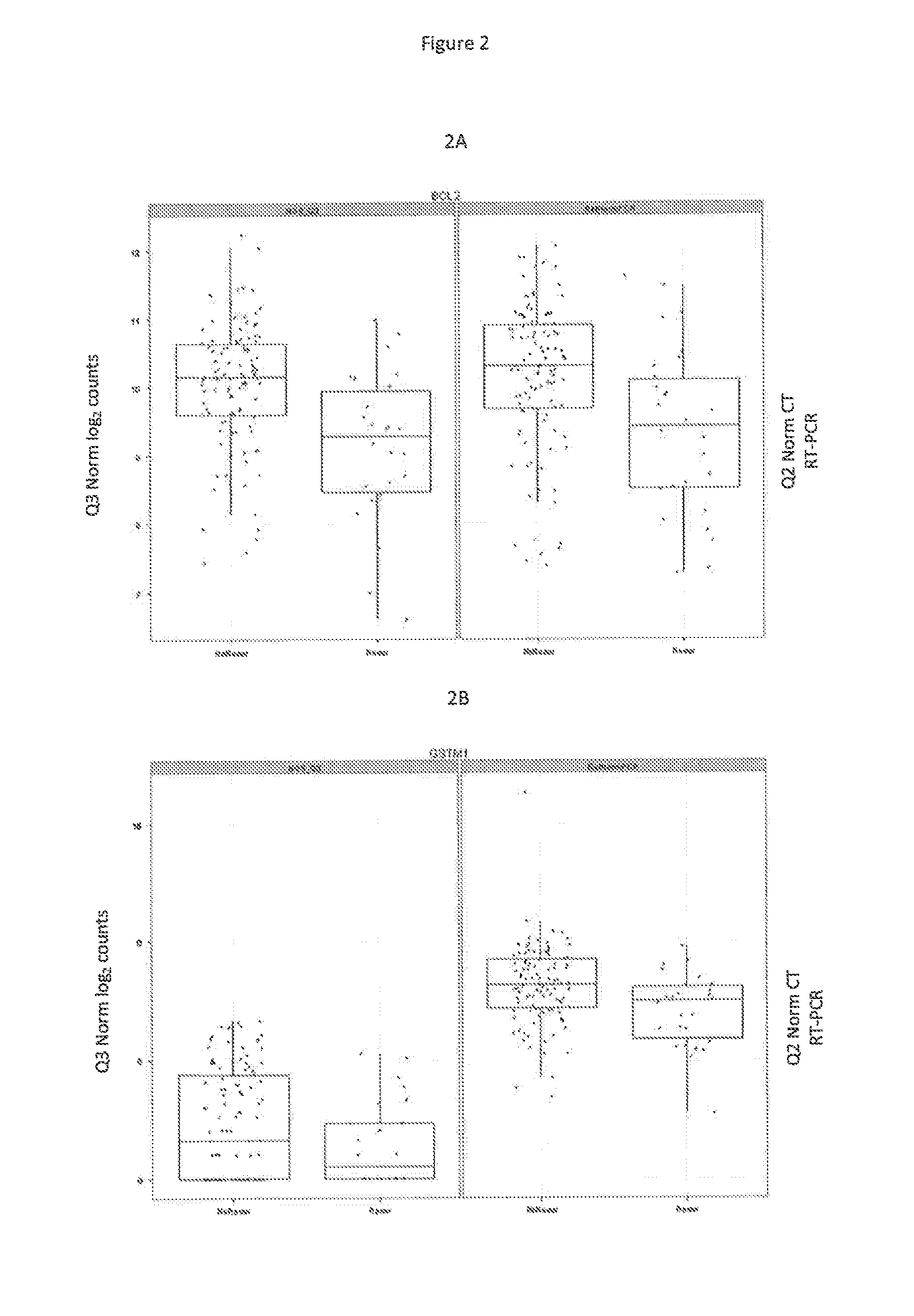
The present invention relates to quantitative molecular indicators that can guide clinical decisions in breast cancer, such as estrogen receptor (ESR1)-positive, lymph node-negative breast cancer. In particular, the invention concerns certain genes, the varied expression of which indicates the likelihood of recurrence of surgically resected breast cancer in patients who are not treated with a therapeutic agent in the adjuvant setting. In addition, the invention concerns the use of quantitative measurement of the expression of certain genes, including the ESR1 gene, that measure as a continuous variable, to determine (a) the likelihood of a beneficial response to the anti-estrogen therapeutic agent, such as tamoxifen; and (b) the potential magnitude of beneficial response to chemotherapy.
Owner:MICROSOFT CORP +2
High temperature thermal therapy of breast cancer
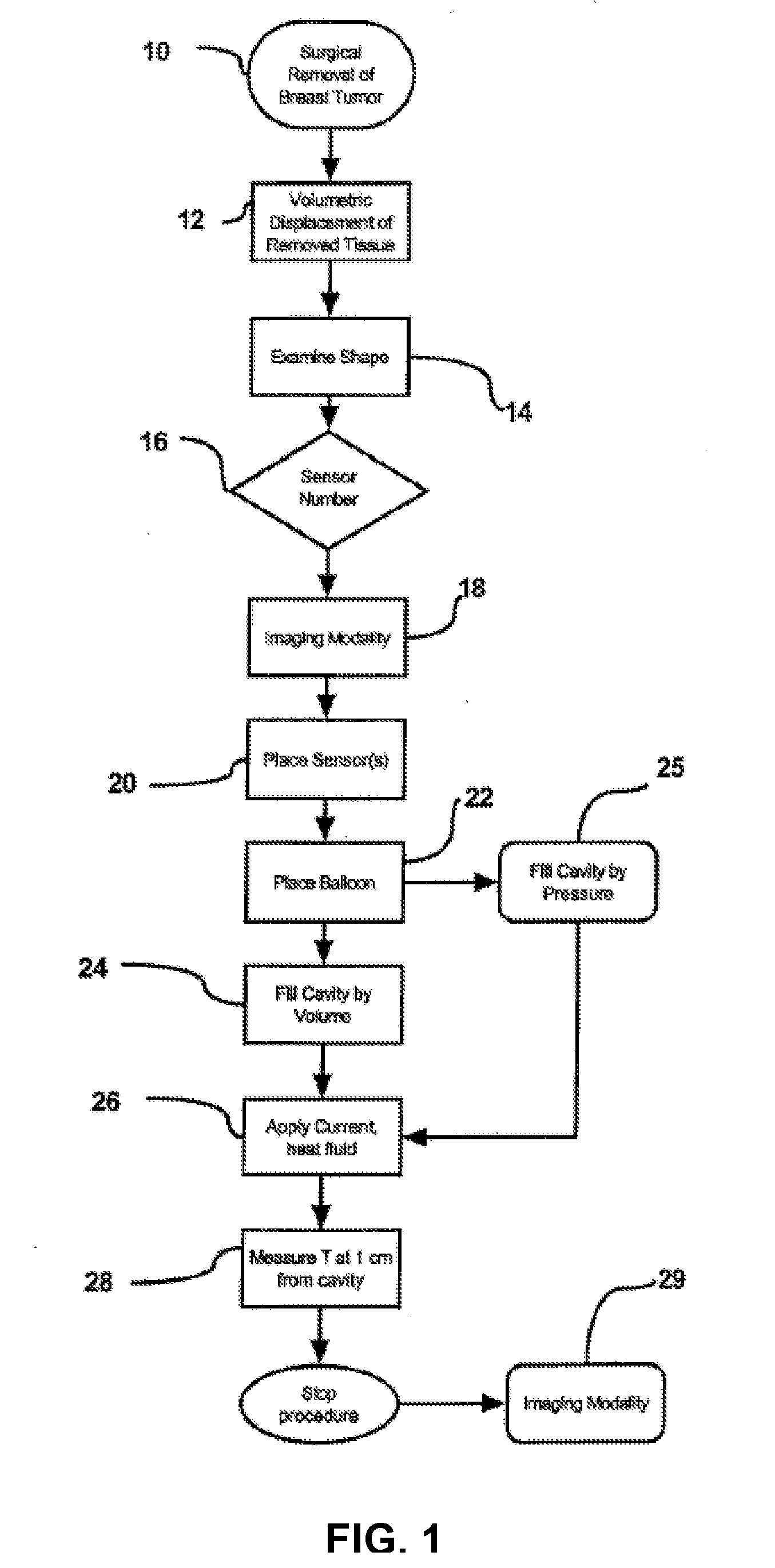
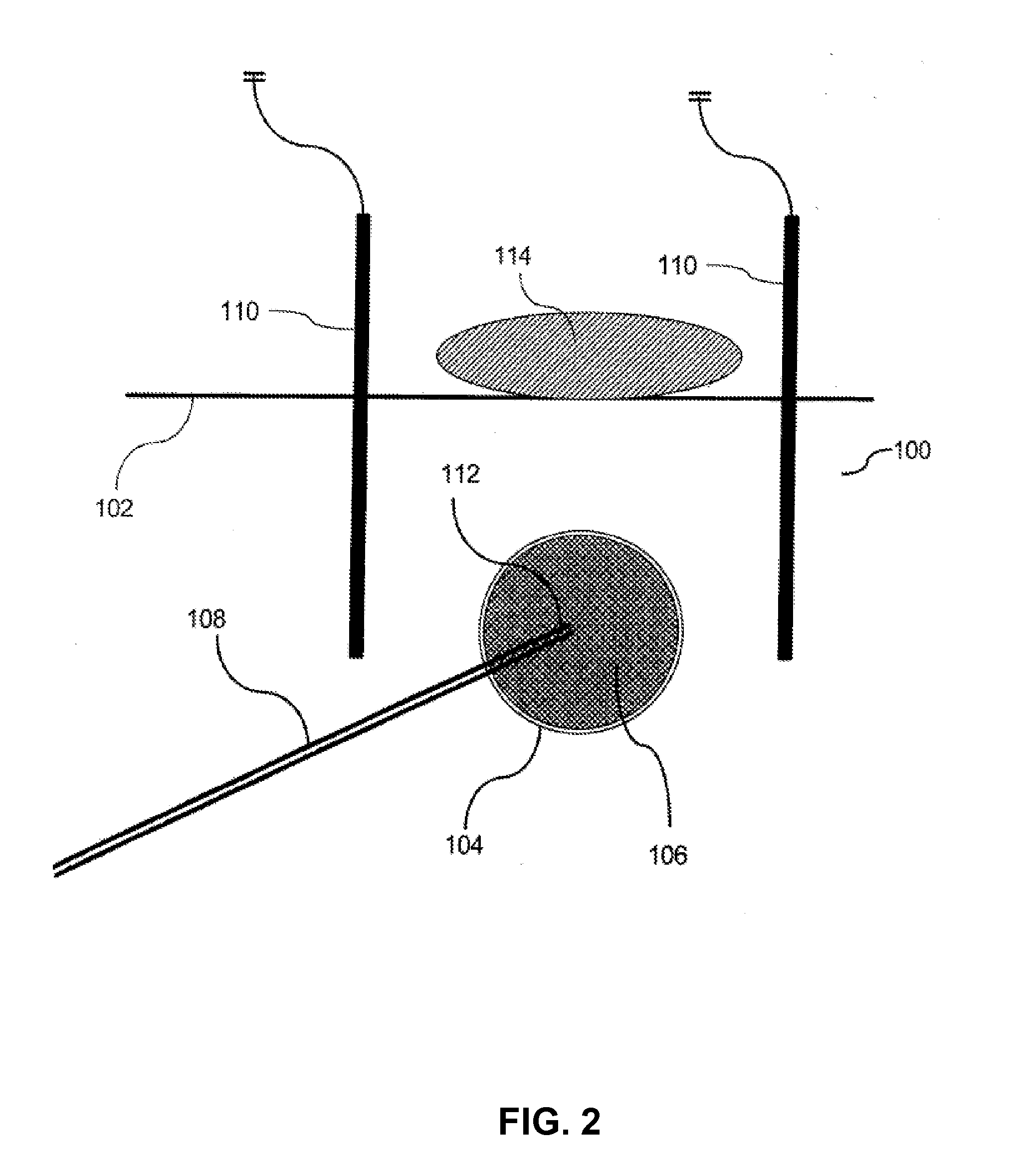
A method is described for preventing breast cancer recurrence after surgical removal of a breast cancer mass. Specifically, the method is thermal treatment of breast tissue surrounding a cavity after lumpectomy for breast cancer. Delivery of a heated fluid is through a balloon catheter to the cavity generated after lumpectomy with the goal of ablating the surrounding tissue, including transformed cells that were not removed through surgery. A balloon and controller were designed for this application.
Owner:THERMATOME
Molecular indicators of breast cancer prognosis and prediction of treatment response
ActiveUS7622251B2Reduce riskMicrobiological testing/measurementProteomicsAdjuvantLymph node negative
The present invention relates to quantitative molecular indicators that can guide clinical decisions in breast cancer, such as estrogen receptor (ESR1)-positive, lymph node-negative breast cancer. In particular, the invention concerns certain genes, the varied expression of which indicates the likelihood of recurrence of surgically resected breast cancer in patients who are not treated with a therapeutic agent in the adjuvant setting. In addition, the invention concerns the use of quantitative measurement of the expression of certain genes, including the ESR1 gene, that measure as a continuous variable, to determine (a) the likelihood of a beneficial response to the anti-estrogen therapeutic agent, such as tamoxifen; and (b) the potential magnitude of beneficial response to chemotherapy.
Owner:MICROSOFT CORP +2
Breast cancer survival and recurrence
InactiveUS20050100933A1Accurate assessmentDetermining prognosisMicrobiological testing/measurementFermentationPatient survivalInvasive breast carcinoma
The invention provides for the identification and use of gene expression profiles, or patterns, with clinical relevance to breast cancer. In particular, the invention provides the identities of genes that are correlated with patient survival and breast cancer recurrence. The gene expression profiles may be embodied in nucleic acid expression, protein expression, or other expression formats and used to predict the survival of subjects afflicted with breast cancer and to predict breast cancer recurrence and. The profiles may also be used in the study and / or diagnosis of breast cancer cells and tissue, including the grading of invasive breast cancer, as well as for the study and / or determination of prognosis of a patient. When used for diagnosis or prognosis, the profiles may be used to determine the treatment of breast cancer based upon the likelihood of life expectancy and recurrence.
Owner:UNIV OF LOUISVILLE RES FOUND INC +1
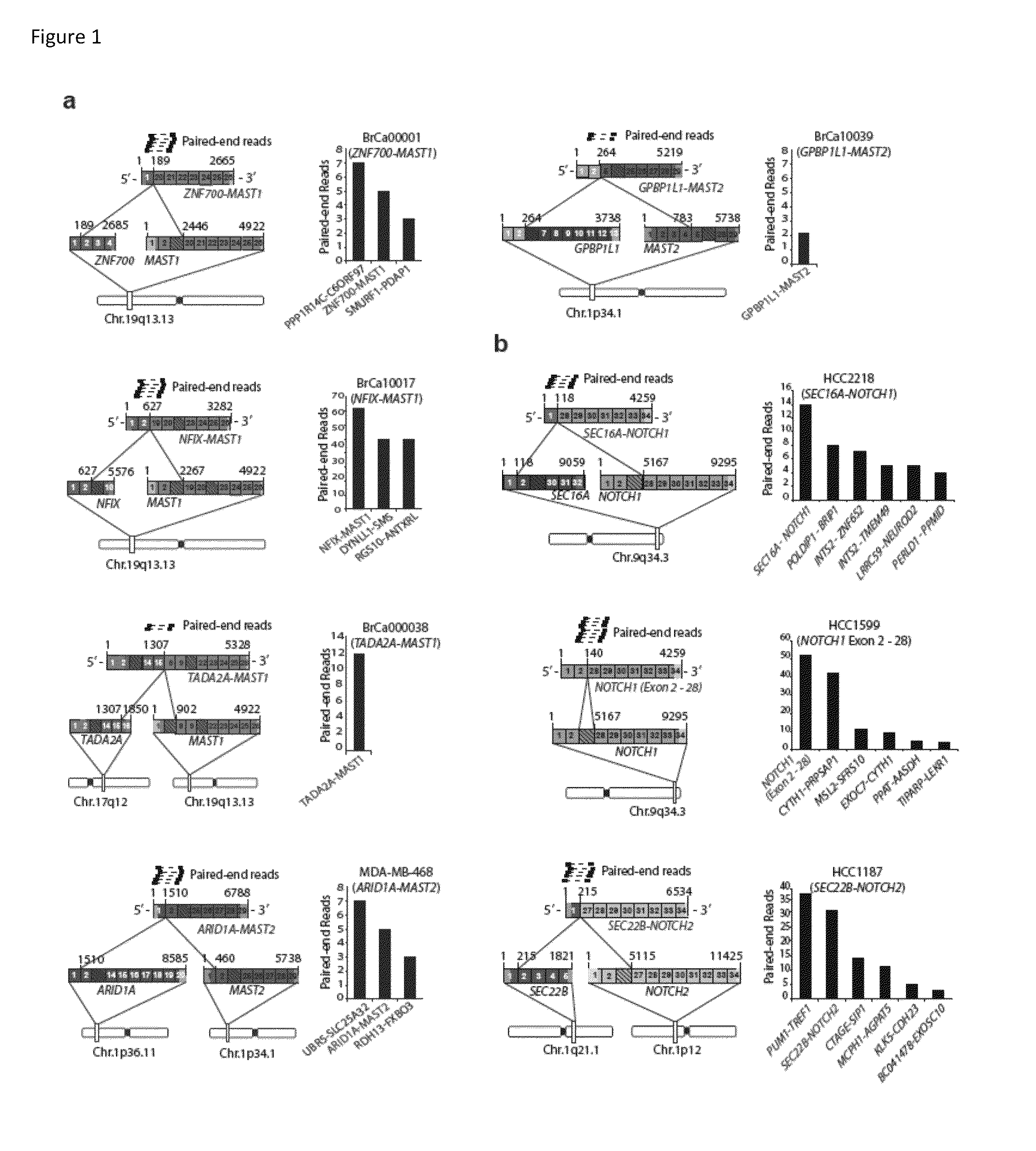
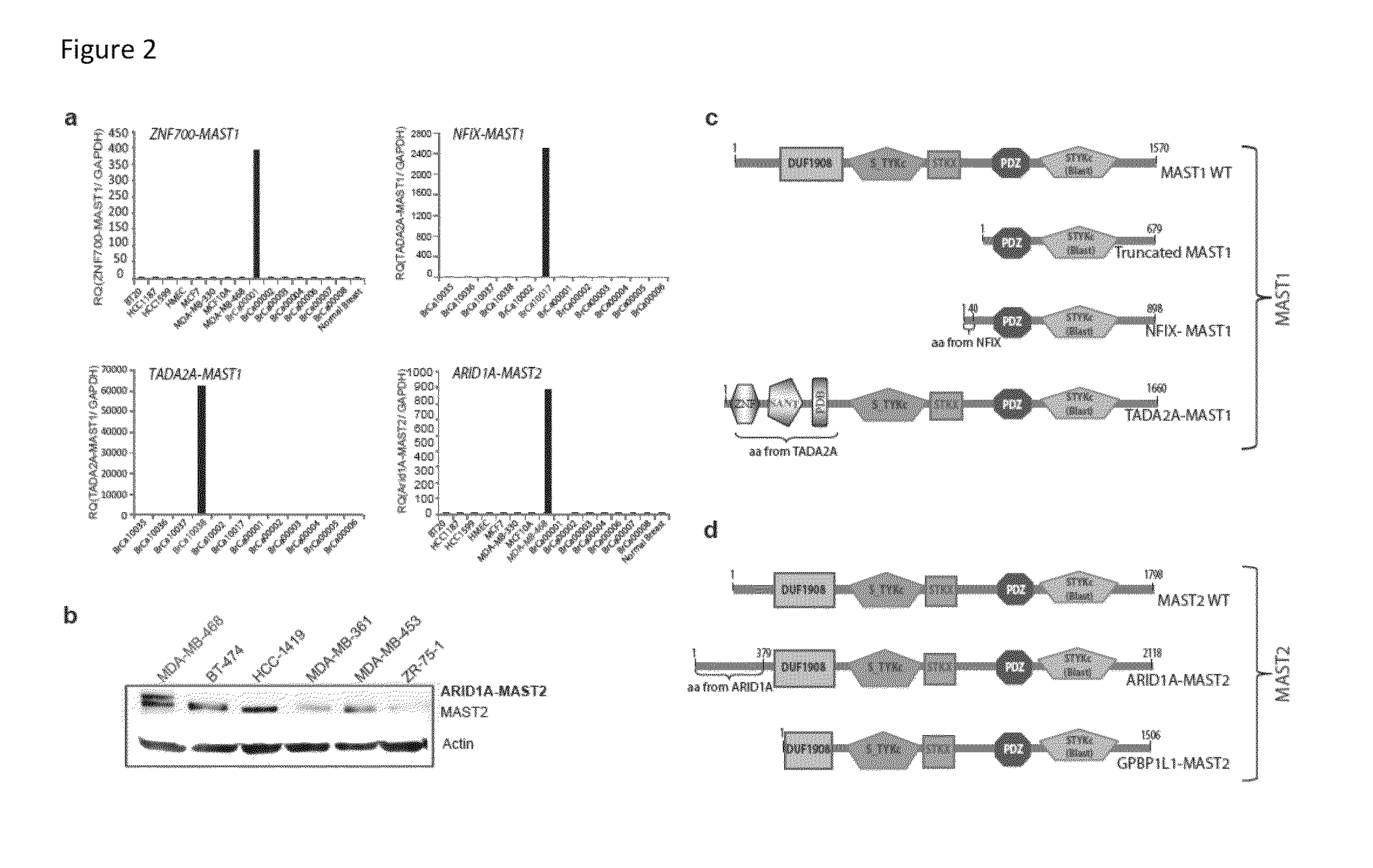
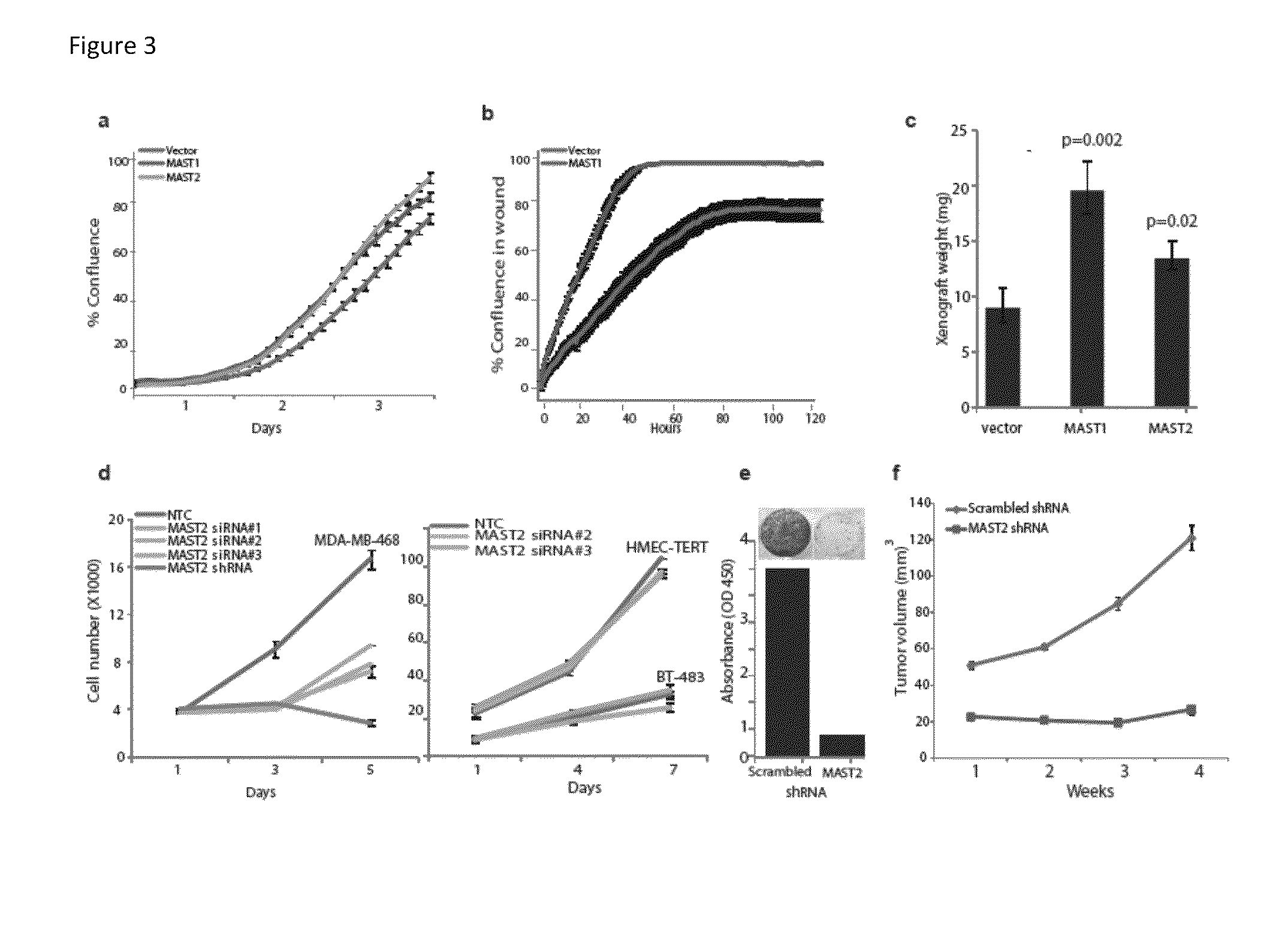
Recurrent gene fusions in breast cancer
The present disclosure relates to compositions and methods for cancer diagnosis, research and therapy, including but not limited to, cancer markers. In particular, the present disclosure relates to gene fusions as diagnostic markers and clinical targets for breast cancer.
Owner:RGT UNIV OF MICHIGAN
Breast cancer recurrent risk assessment 21 gene detection primer and application thereof
InactiveCN107058523ASimple and fast operationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorAdjuvant chemotherapy
The invention relates to a breast cancer recurrent risk assessment 21 gene detection primer and application thereof. The breast cancer recurrent risk assessment 21 gene detection primer comprises a nucleotide sequence as shown in an SEQ ID NO.: 1 to 42, and belongs to the technical field of molecular detection. The detection primer provided by the invention utilizes the advantage of fluorescent quantitation, and adopts fluorescent quantitation polymerase chain reaction to carry out amplification detection on 21 genes for breast cancer recurrent risk assessment, so that whether clinical measurements such as adjuvant chemotherapy are needed or not is considered. A kit provides a PCR (Polymerase Chain Reaction) amplification primer sequence of the 21 genes for breast cancer recurrent risk assessment, and the primer is applicable to detecting ER positive patients, HER2 negative patients, axillary node negative patients, moderately and poorly differentiated patients with the tumor size being 0.6 to 1.0cm and adverse prognostic factors, or patients with the tumor size is larger than 1cm, has the advantages of high sensitivity and specificity, stability, promptness, convenience in operation and the like, can well meet the clinical application of breast cancer recurrent risk assessment, and can be beneficial for reducing invalid medication administration, improving medication administration accuracy, and reducing financial burdens of the patients.
Owner:温州迪安医学检验所有限公司 +1
Method for breast cancer recurrence prediction under endocrine treatment
InactiveUS20130065786A1Reliable predictionNucleotide librariesMicrobiological testing/measurementDisease outcomeEndocrine therapy
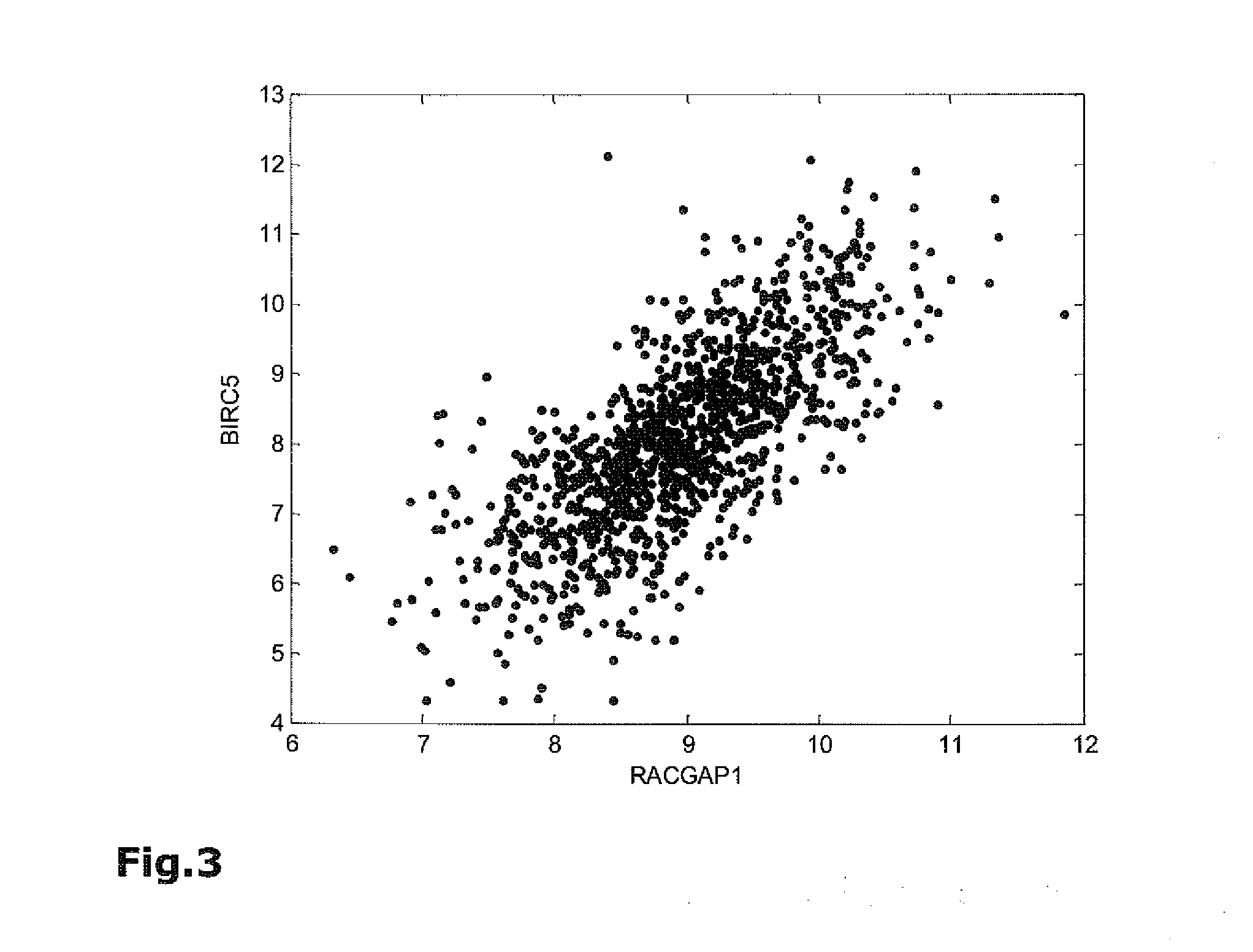
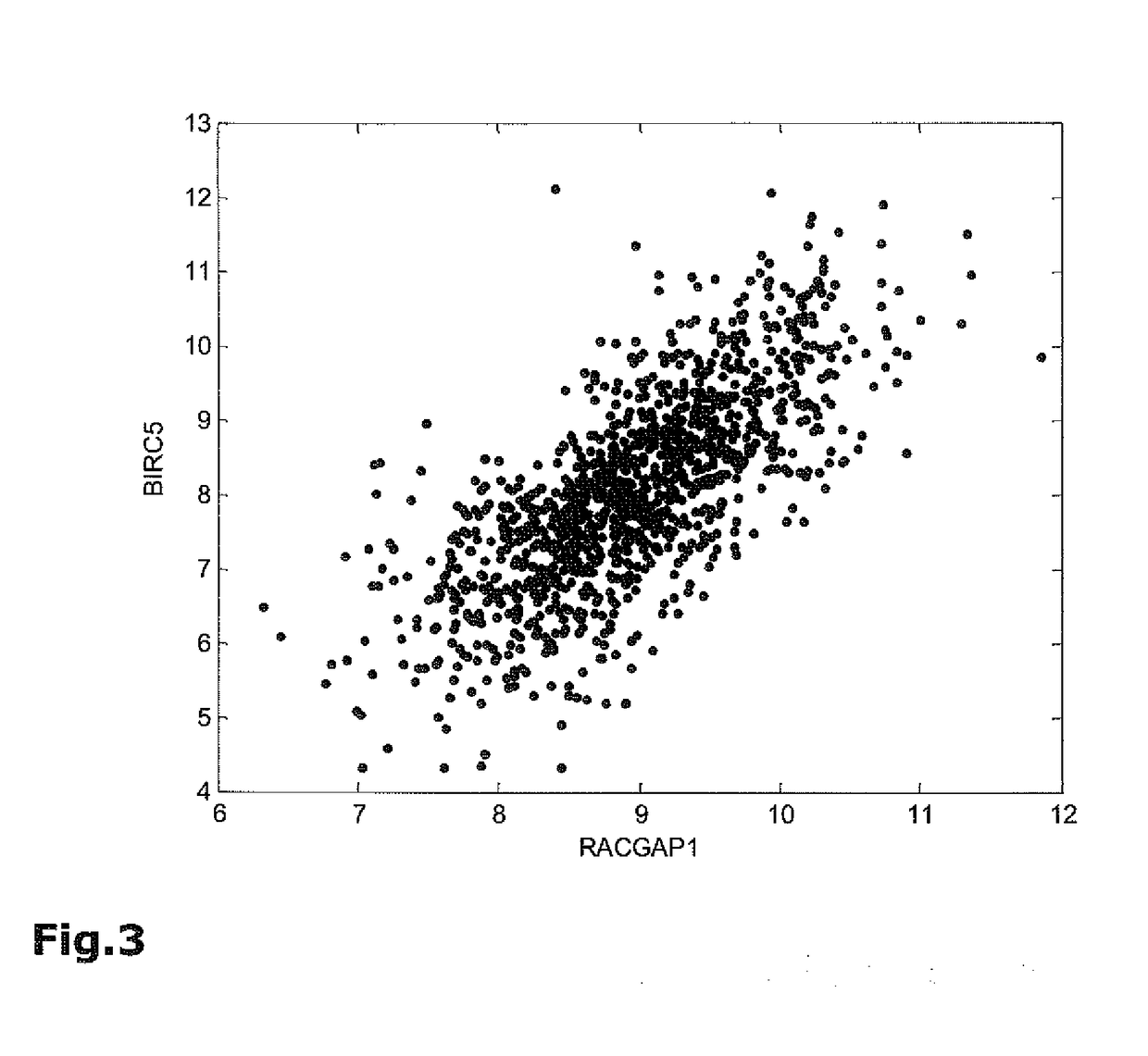
The present invention relates to methods, kits and systems for the prognosis of the disease outcome of breast cancer, said method comprising:(a) determining in a tumor sample from said patient the RNA expression levels of at least 2 of the following 9 genes: UBE2C, BIRC5, RACGAP1, DHCR7, STC2, AZGP1, RBBP8, IL6ST, and MGP(b) mathematically combining expression level values for the genes of the said set which values were determined in the tumor sample to yield a combined score, wherein said combined score is indicative of a prognosis of said patient; and kits and systems for performing said method.
Owner:SIVIDON DIAGNOSTICS
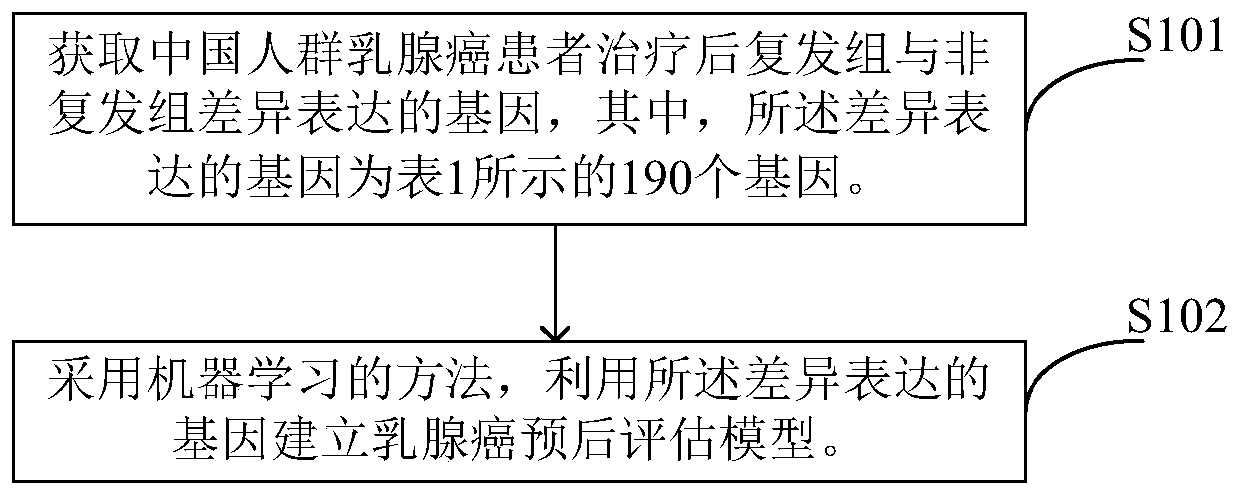
Breast cancer prognosis evaluation model and establishing method thereof
InactiveCN110656173ALess rapid disease progressionNo disease progressionMicrobiological testing/measurementBiostatisticsSide effectIndividualized treatment
The invention provides a breast cancer prognosis evaluation model and an establishing method thereof. Differentially expressed genes are screened by selecting Chinese population as the research objectand through an RNA-seq method, the genes closely related to recurrence are screened out, and thus the prediction model suitable for the breast cancer recurrence risk of the Chinese population is obtained; and the model comprises the 190 genes which are differentially expressed in recurrence groups and non-recurrence groups and shown in the tablet 1. The model can provide more accurate individualized treatment effect prediction and ten-year recurrence risk prediction for early-stage breast cancer patients of the Chinese population, thus disease progression does not quickly occur in the high-risk population due to the lack of postoperative adjuvant chemotherapy, and the bodies of the low-risk population do not bear unnecessary chemotherapy toxic and side effects due to overtreatment either.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
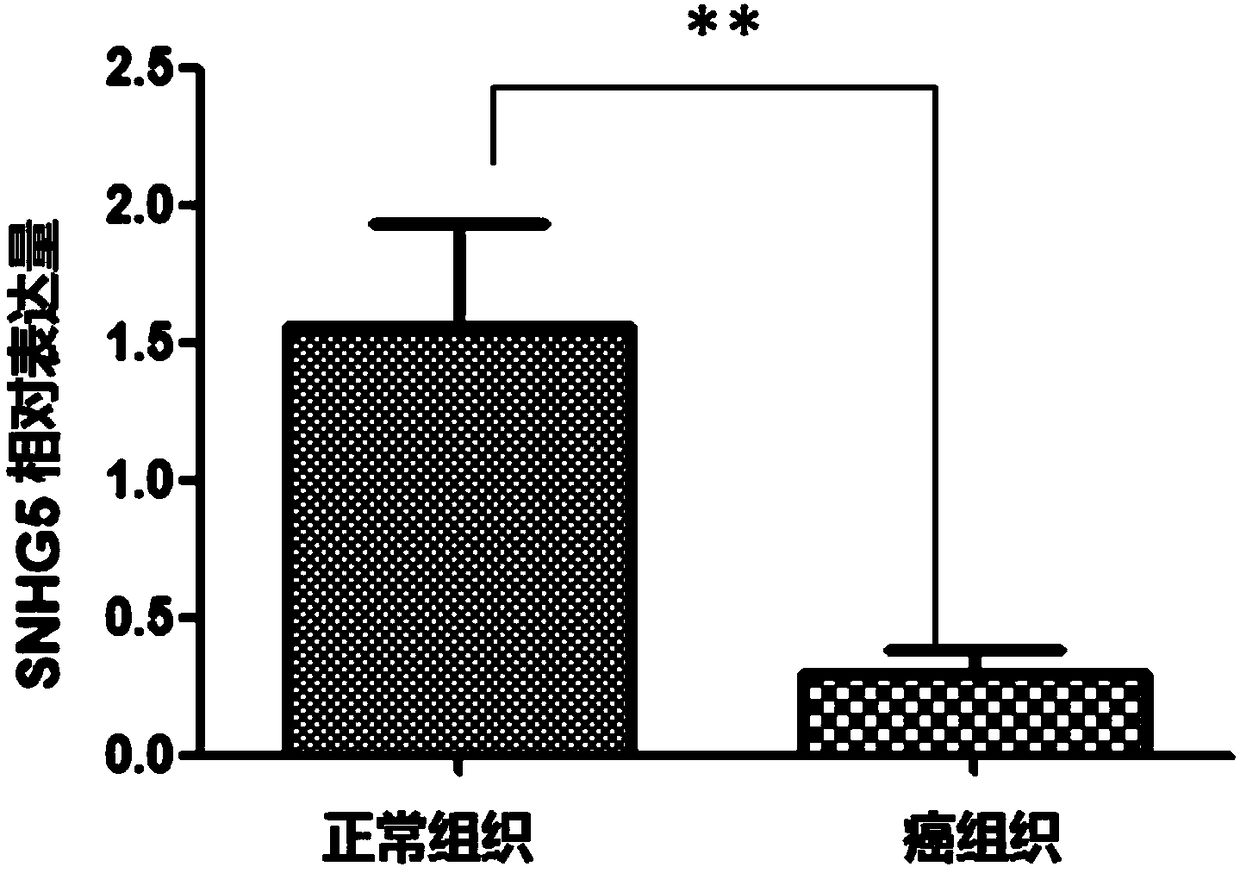
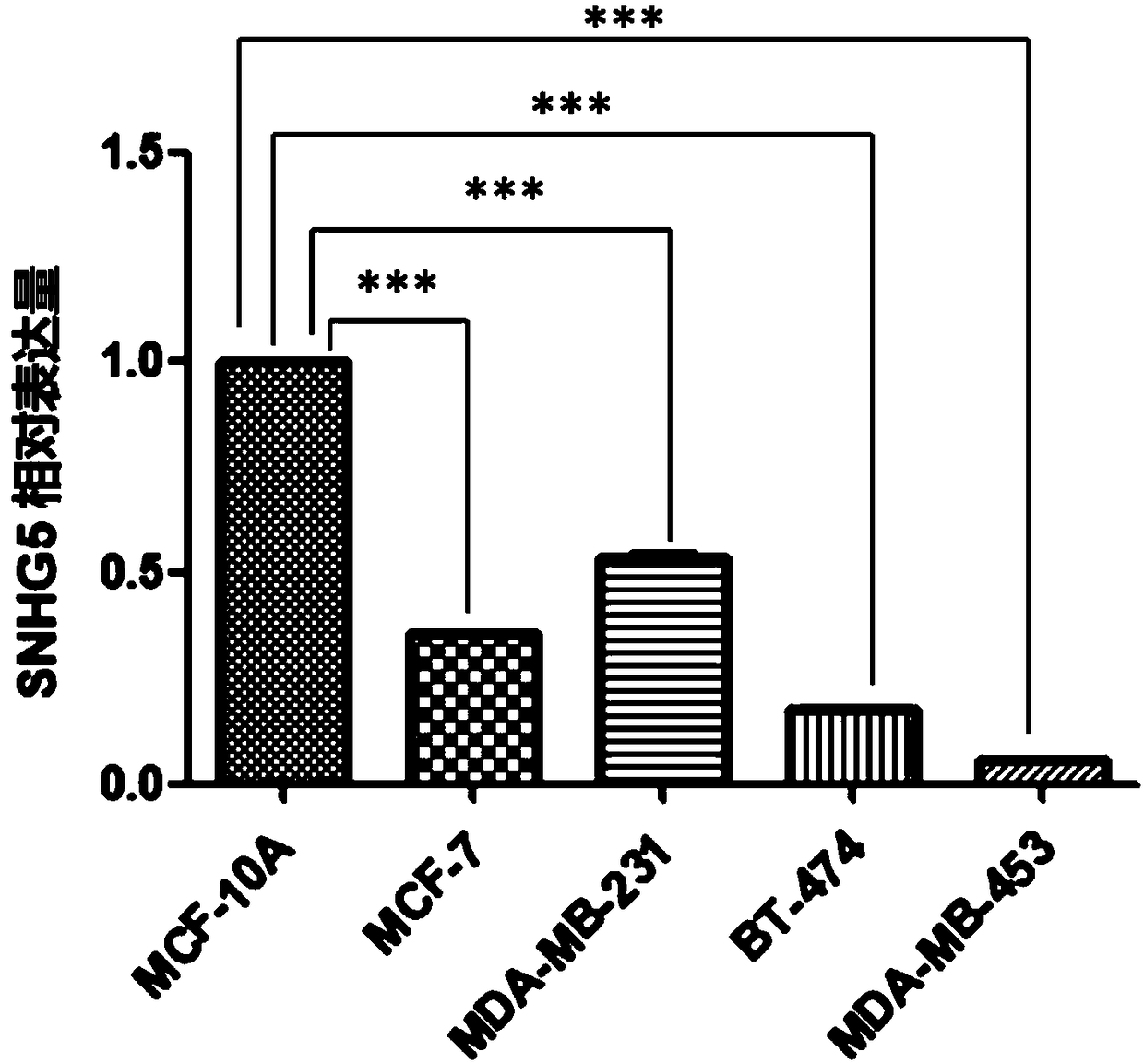
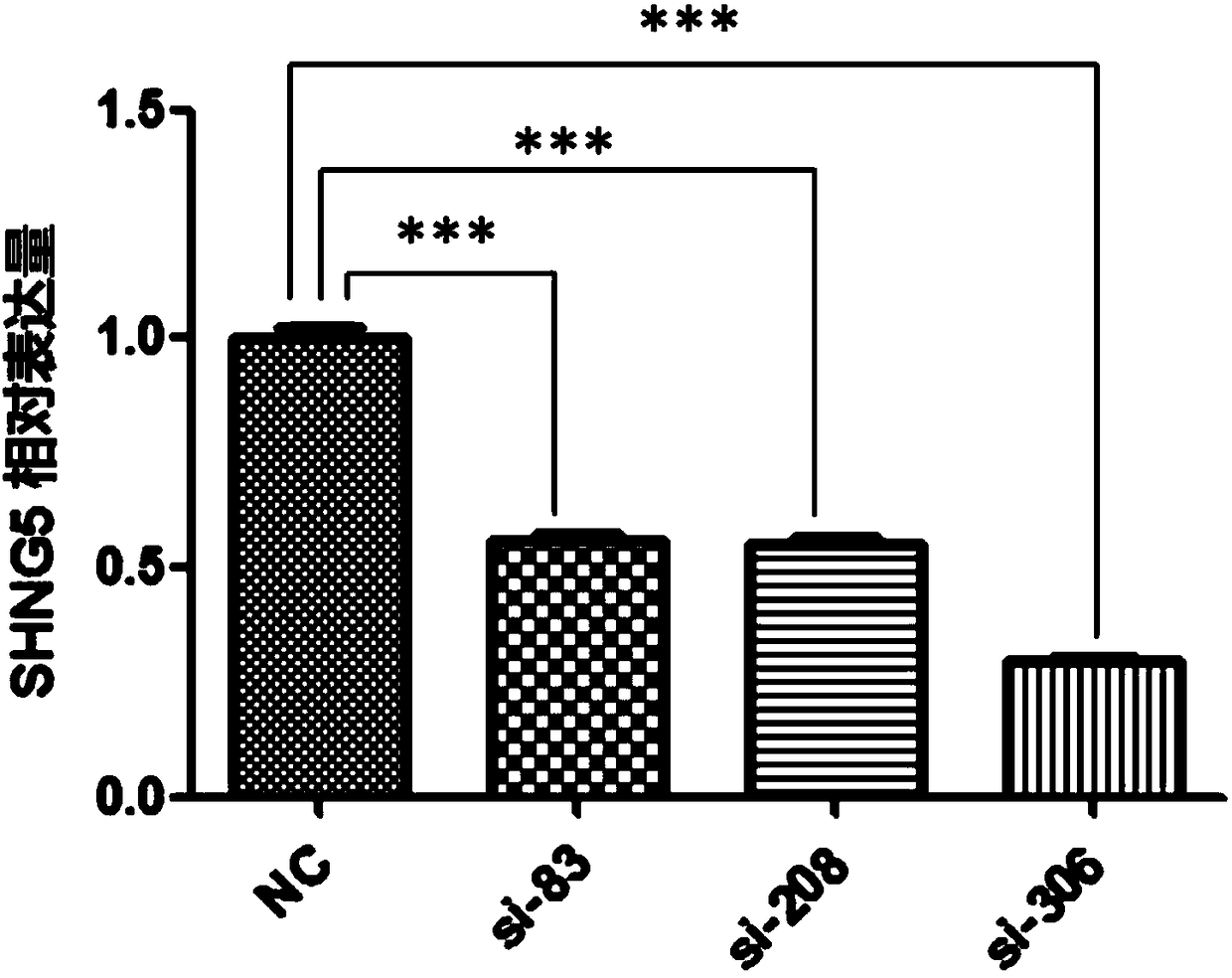
Application of SNHG5 (small nucleolar RNA host gene 5) in diagnosis, evaluation and efficacy prediction of breast cancer
InactiveCN108467891AHigh degree of malignancyMicrobiological testing/measurementApoptosisWilms' tumor
The invention discloses an application of SNHG5 (small nucleolar RNA host gene 5) in diagnosis, evaluation and efficacy prediction of breast cancer. It is found that lncRNA SNHG5 has significant low expression in breast cancer tissue and breast cancer cell lines, knockout of the lncRNA SNHG5 gene can enhance proliferation and migration of breast cancer cells and inhibit apoptosis, and thus, the expression level of long-chain non-coding RNA SNHG5 is closely related with diagnosis, transfer and relapse of tumor, patient prognosis and neoadjuvant chemotherapy effect are closely related, which indicates that the lncRNA SNHG5 gene can be used as a marker for diagnosis, prognosis evaluation or effect prediction. A breast cancer cell model which has low expression of SNHG5 and is established by transfecting breast cancer cell lines with small interference RNA can be used in a preparation method of a preparation for preventing relapse and transfer of breast cancer.
Owner:TONGDE HOSPITAL OF ZHEJIANG PROVINCE
Method of predicting breast cancer prognosis
InactiveUS20140296085A1Microbiological testing/measurementLibrary screeningIntergenic SequenceOncology
The present invention relates to biomarkers associated with breast cancer prognosis. These biomarkers include coding transcripts and their expression products, as well as non-coding transcripts, and are useful for predicting the likelihood of breast cancer recurrence in a breast cancer patient, The present invention also relates to a novel method of identifying intergenic sequences that correlate with a clinical outcome.
Owner:GENOMIC HEALTH INC
Diagnosis, Prognosis and Prediction of Recurrence of Breat Cancer
InactiveUS20090222387A1Improve accuracyDigital computer detailsBiostatisticsTissue sampleBreast cancer subtype
The present invention relates to methods and compositions for the diagnosis, prognosis, and prediction of breast cancer. More specifically, the invention relates to classification of breast cancer tissue samples based on measuring the expression of a set of marker genes. The set is useful for the identification of clinically important breast cancer subtypes. Methods are disclosed for prediction, diagnosis and prognosis of breast cancer.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS GMBH
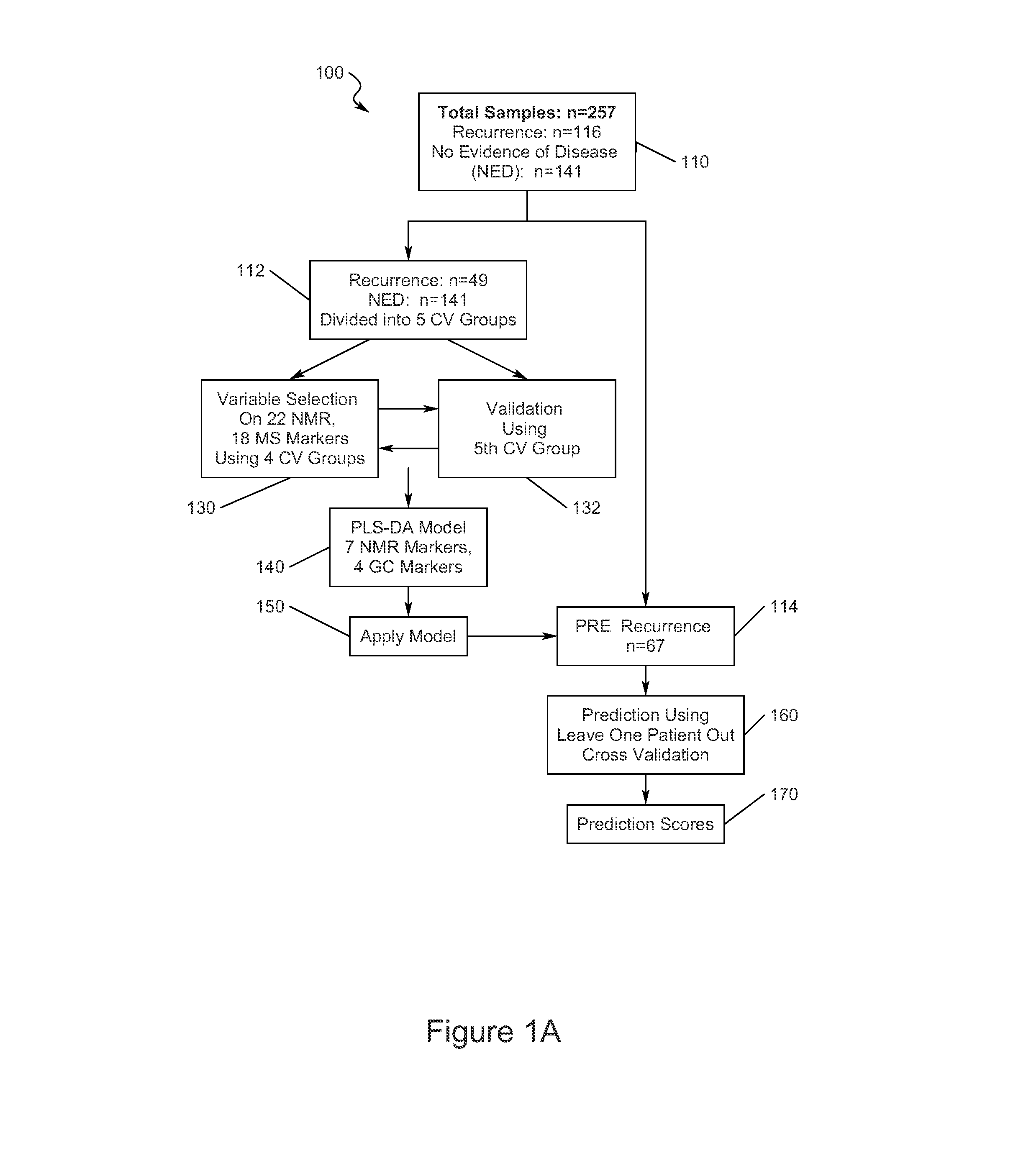
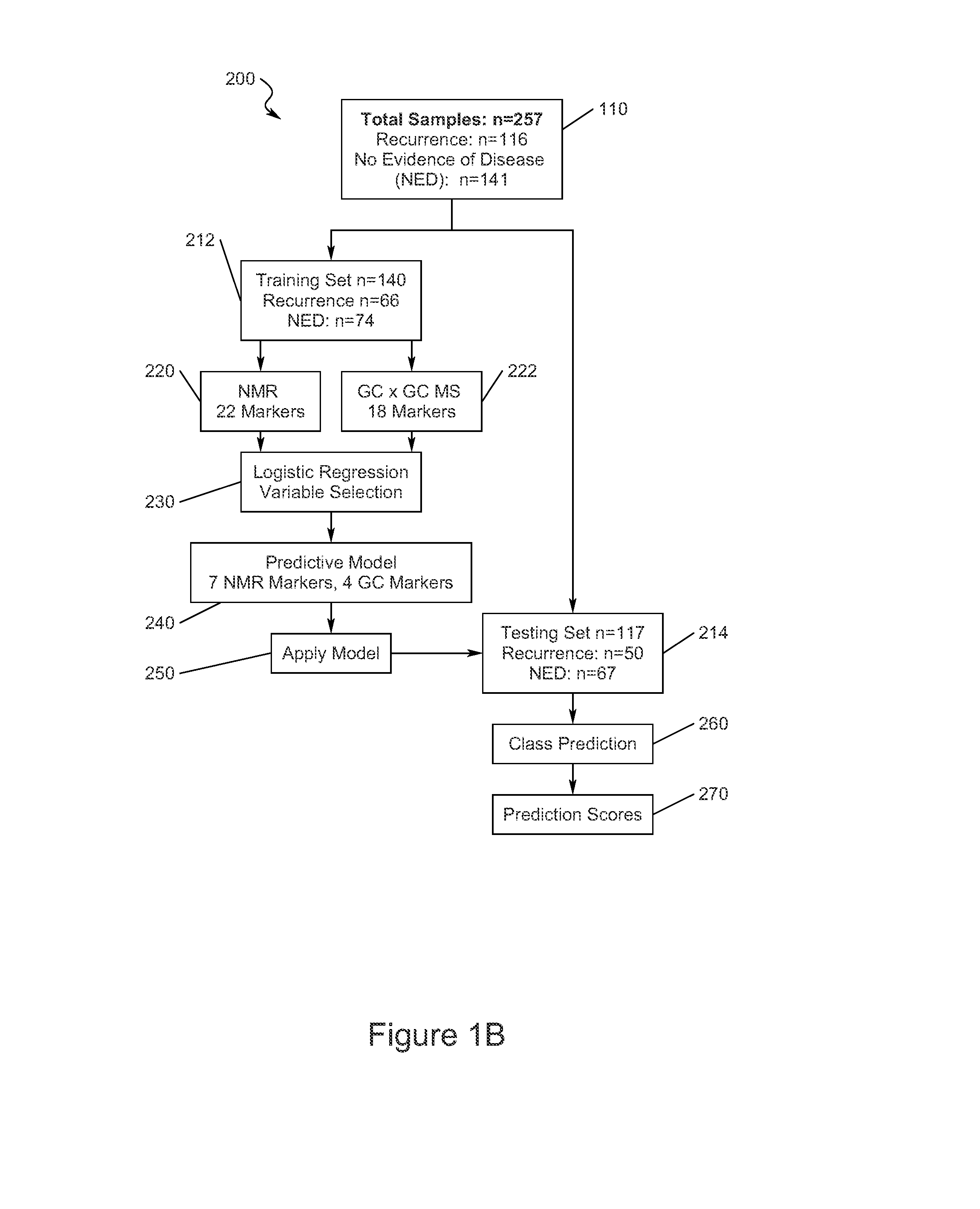
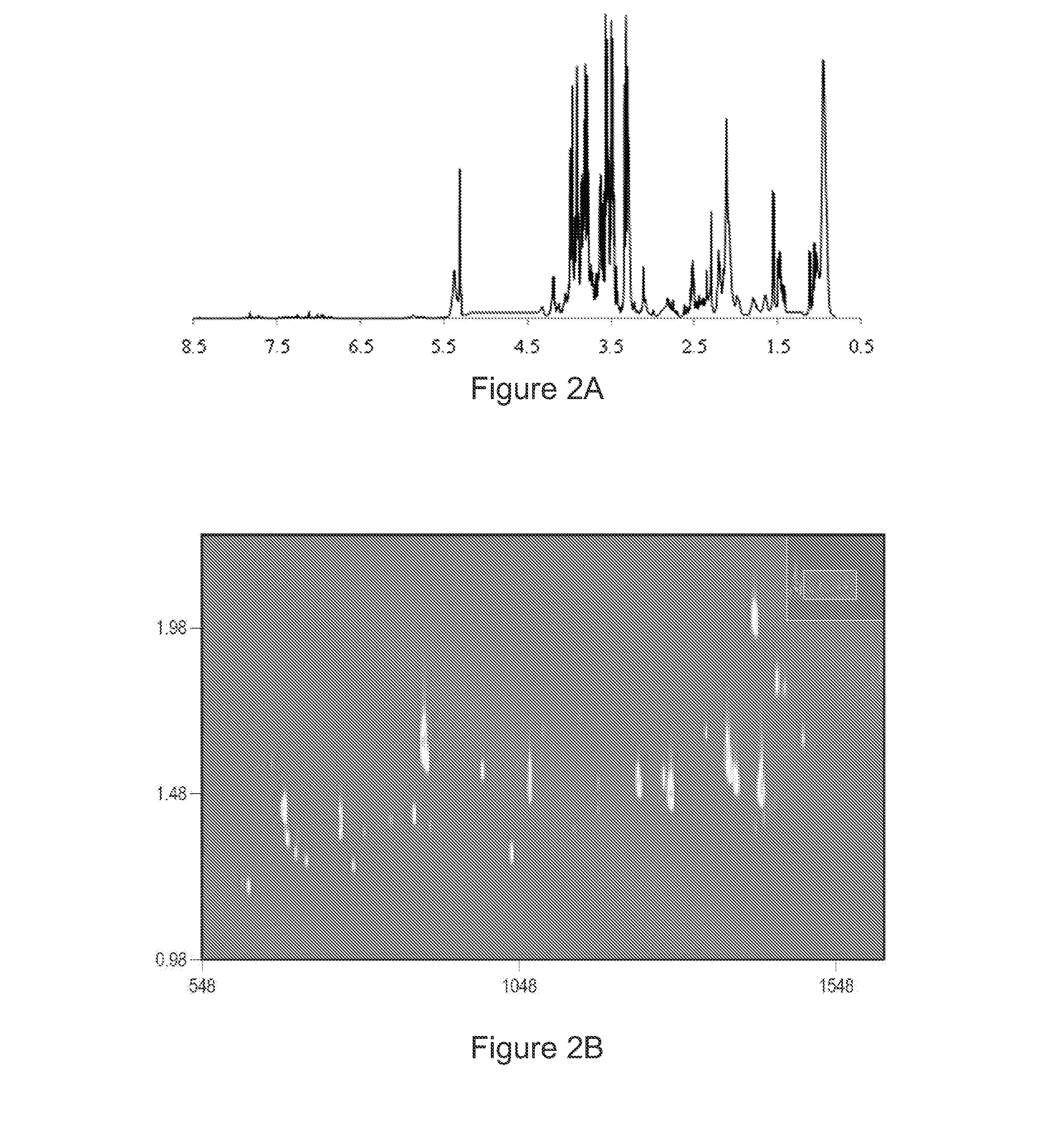
Early detection of recurrent breast cancer using metabolite profiling
InactiveUS20130023056A1High degree of sensitivityHigh degree of specificityComponent separationOrganic compound preparationDiseaseDiagnostic test
A monitoring test for recurrent breast cancer with a high degree of sensitivity and specificity is provided that detects the presence of a panel of multiplicity of biomarkers that were identified using metabolite profiling methods. The test is capable of detecting breast cancer recurrence about a years earlier than current available monitoring diagnostic tests. The panel of biomarkers is identified using a combination of nuclear magnetic resonance (NMR) and two dimensional gas chromatography-mass spectrometry (GC×GC-MS) to produce the metabolite profiles of serum samples. The NMR and GC×GC-MS data are analyzed by multivariate statistical methods to compare identified metabolite signals between samples from patients with recurrence of breast cancer and those from patients having no evidence of disease.
Owner:PURDUE RES FOUND INC
Hormone receptor-positive breast cancer recurrence monitoring gene mutation library construction method
The invention discloses a hormone receptor-positive breast cancer recurrence monitoring gene mutation library construction method, which is characterized that the library covers the total 1357 somatic cell mutations on human genes such as KRAS, JAK3, AKT1, CREBBP, PTEN, RB1, TP53, CTNNB1, TSC2, TSC1, ERBB2, PIK3CA, SF3B1, JAK2, CDH1, SMAD4, GATA3, MAP2K4, MAP3K1 and ESR1. According to the present invention, a plurality of the target sequences are subjected to single tube amplification with the construction method to rapidly complete the library construction, wherein the whole library construction process takes only 2-3 h and the manual time only needs 45 min, such that the difficulty that the multi-gene and multi-target detection of somatic cells on the basis of the small amount of the peripheral blood sample is required in the clinical breast cancer recurrence monitoring is required can be effectively solved, and the lost is low.
Owner:XIAMEN SPACEGEN BIOTECH CO LTD
Multi-detection kit for breast cancer 21 gene
PendingCN110129435AExtend the life cycleReduce contributionMicrobiological testing/measurementDNA/RNA fragmentationGynecologyMedical expenses
The invention belongs to the field of biomedicine, relates to a tumor detection kit, and in particular, relates to a detection kit for risk evaluation of breast cancer recurrence. The invention relates to a multi-detection kit for a breast cancer 21 gene; according to the multi-detection kit for the breast cancer 21 gene, provided is a method for multiple detection of the breast cancer 21 gene; the method has the advantages of small used sample size, fast speed, low cost and the like, and is used for serving the majority of breast cancer patients; by early diagnosis and early treatment, required contribution is made for prolonging the life cycle of patients with breast cancer and reducing unnecessary medical expenses.
Owner:深圳塔歌生物技术有限公司
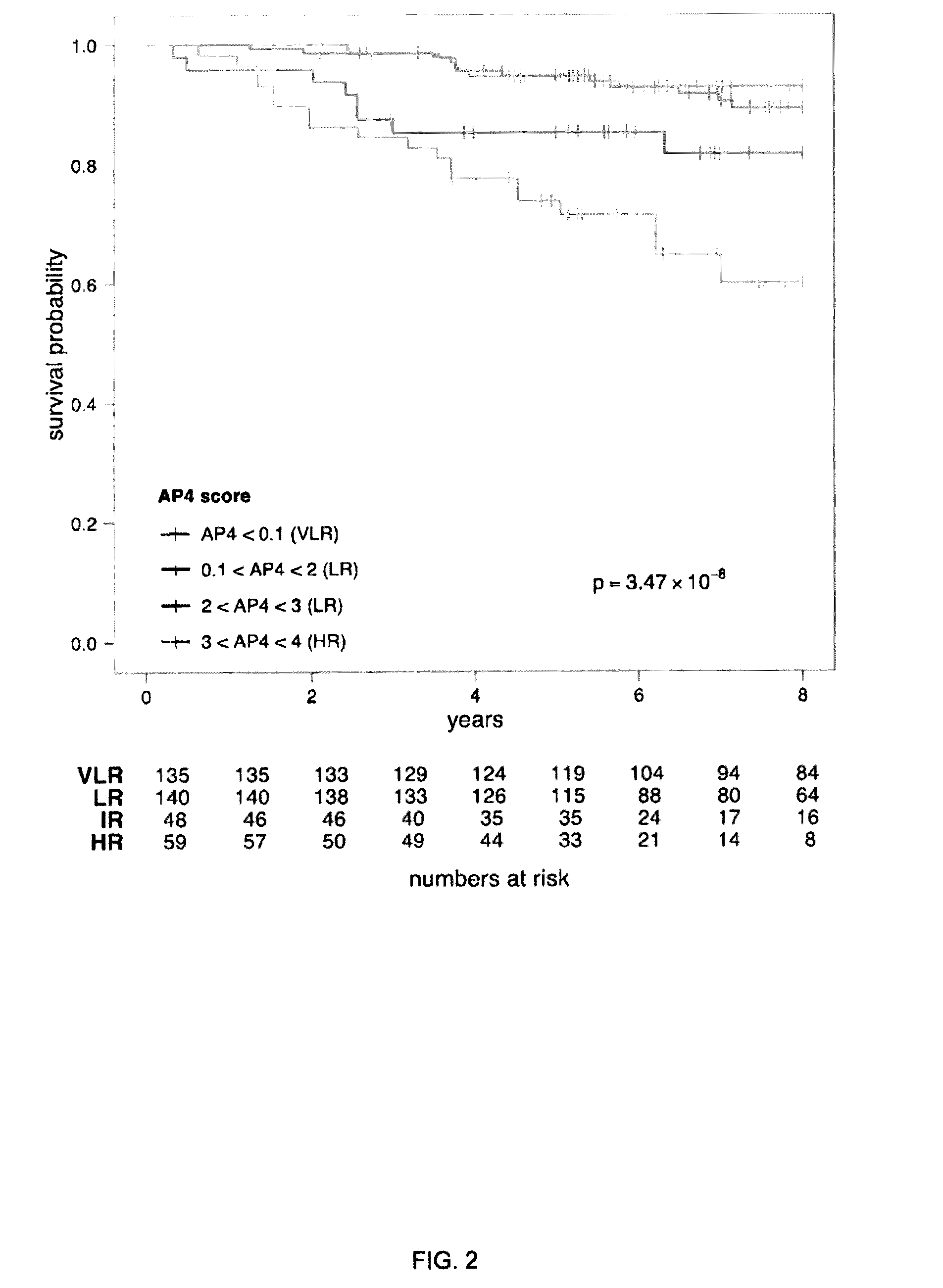
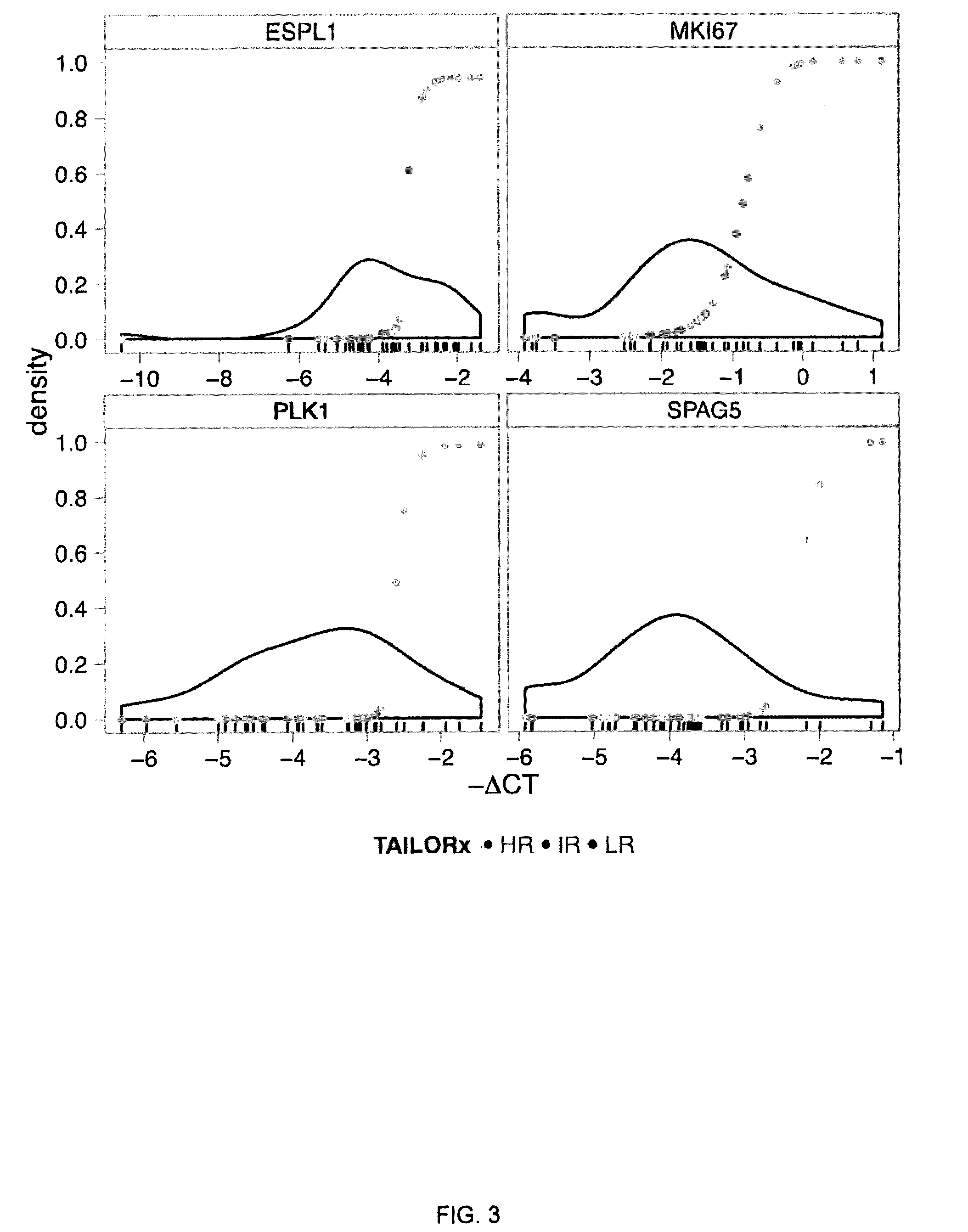
Breast cancer prognostication and screening kits and methods of using same
ActiveUS20160333413A1Improved and more accurate screening and prognostic testsHigh riskMicrobiological testing/measurementSpecial data processing applicationsBiomarker panelRegimen
A genetic biomarker panel is provided for prognosing late onset ER+ breast cancer relapse, in a breast cancer survivor patient. Kits are also provided for measuring levels or the presence of an identified panel of genetic biomarkers. Methods are also provided for identifying a breast cancer survivor patient at a relatively high risk of suffering a breast cancer relapse within 8 years of diagnosis, and therefore suitable for treatment with an aggressive chemotherapeutic regimen. The method may also be used for identifying a breast cancer survivor patient not at high risk of suffering a breast cancer relapse within 8 years of diagnosis, and thus not suitable for treatment with an aggressive chemotherapeutic regimen. The genetic biomarker panel includes an oligonucleotide / nucleic acid sequence specific for the following genes: MKI67, SPAG5, ESPL1, PLK1, or a genetic panel for MKI67, SPAG5, ESPL1, PLK1 and PGR.
Owner:INDIANA UNIV RES & TECH CORP +1
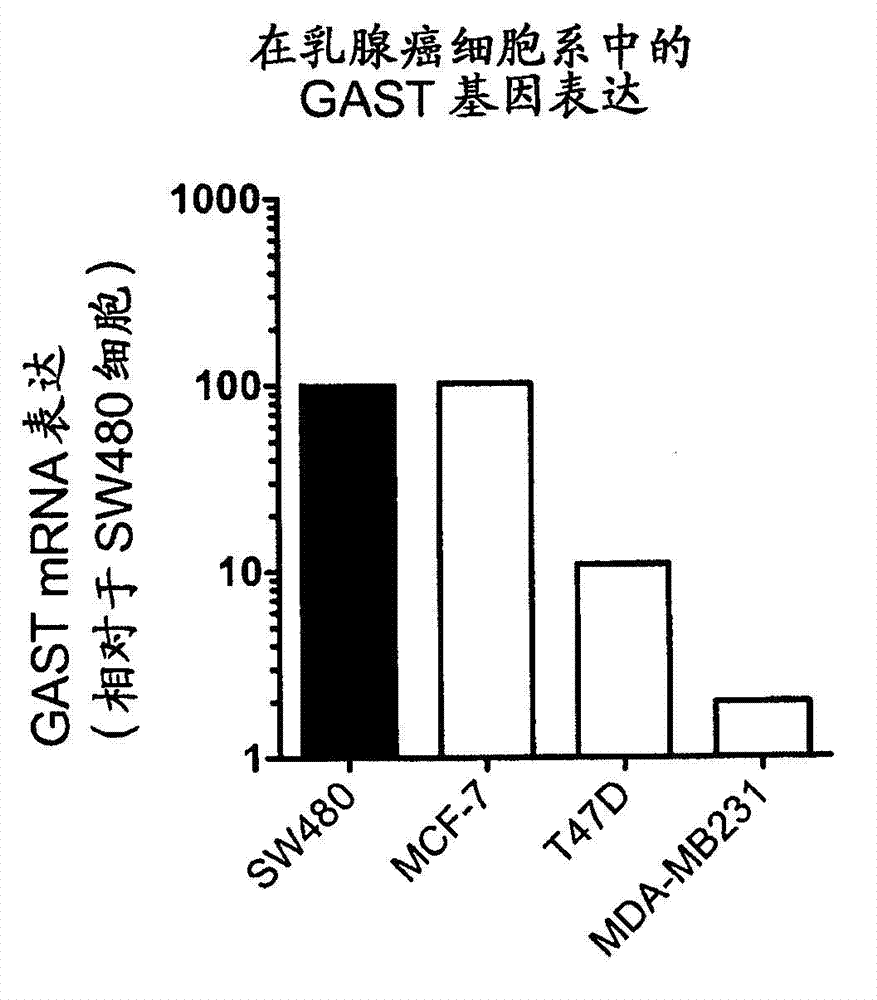
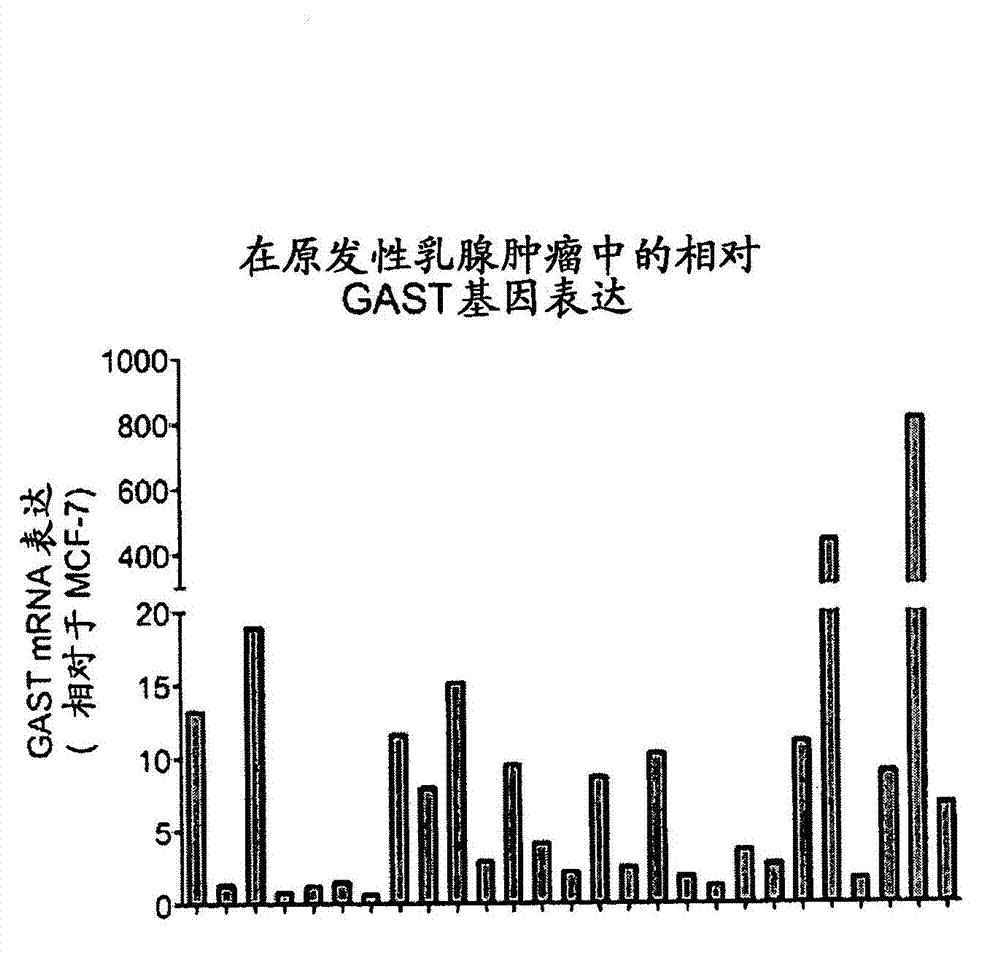
Methods for treating breast cancer
The present disclosure is directed to methods of treating and preventing breast cancer or recurrence of breast cancer with compositions comprising anti-progastrin antibodies.
Owner:LES LAB SERVIER +2
Method for In Vitro Diagnosing and Prognosing of Triple Negative Breast Cancer Recurrence
ActiveUS20160291021A1Reliable and rapid detectionGood indicationDisease diagnosisBiological testingOncologyTriple-negative breast cancer
The present invention is in the technical field of breast cancer management, and more particularly relates to the diagnosis and / or prognosing of triple-negative breast cancer (TNBC). The invention is more particularly based on the finding that specific biomarkers are abberantly expressed in patients suffering from a triple-negative breast cancer recurrence, and are highly related to the aggressiveness of this disease, and thus to survival of said patient.
Owner:INSTITUT DE CANCEROLOGIE DE LOUEST +2
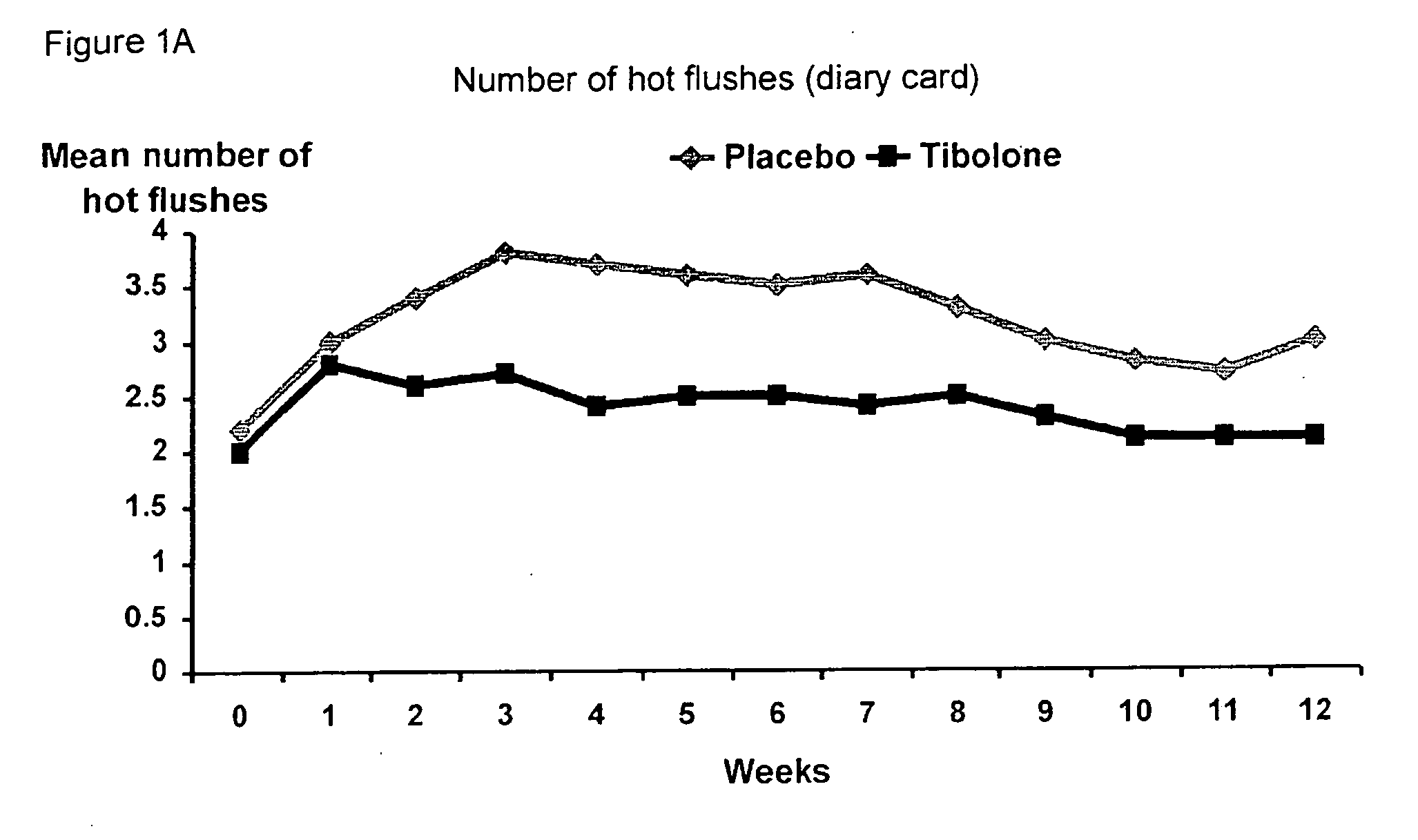
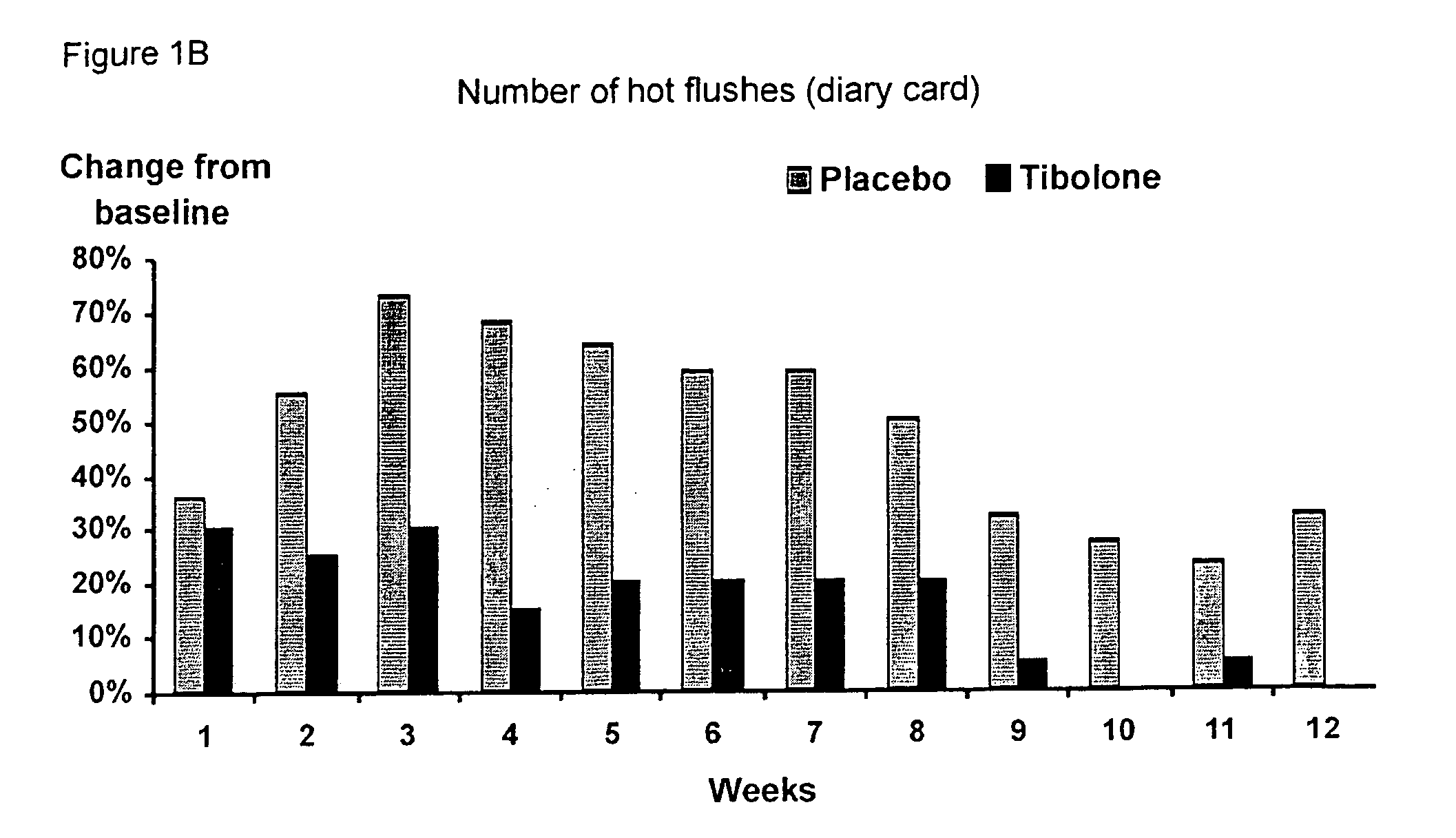
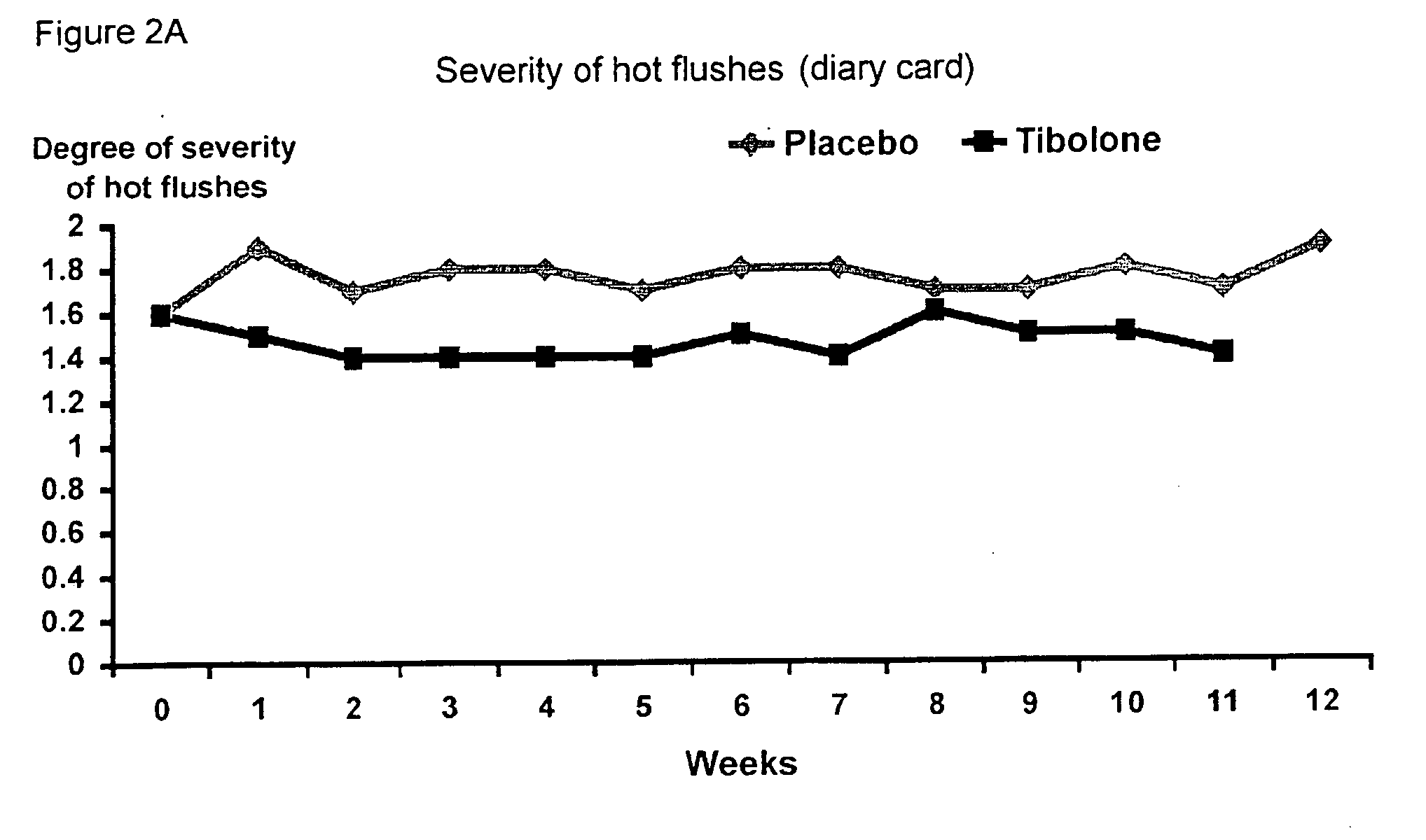
Treatment of post-menopausal complaints in breast cancer patients comprising tibolone and a serm
The subject invention provides a use of tibolone and a SERM for the manufacture of a medicine for the treatment of an estrogen-deficiency related complaint and for the prevention of a recurrence of breast cancer in females suffering from, or at risk for breast cancer that exhibit the estrogen-deficiency related complaint.
Owner:AKZO NOBEL NV
Application of TCP1 protein in preparation of postoperative breast cancer prognosis evaluation kit, postoperative breast cancer prognosis evaluation kit and method
The invention provides the application of the TCP1 protein in the preparation of a breast cancer postoperative prognosis evaluation kit, a breast cancer prognosis evaluation kit and a method. The present invention uses an immunohistochemical method to detect the relative expression of TCP1 protein in breast cancer tissue, so as to judge the risk of breast cancer recurrence and metastasis in breast cancer patients. The beneficial effects of the present invention are mainly reflected in: the present invention provides the application of TCP1 protein in the preparation of breast cancer postoperative prognosis assessment kit, suggesting that the protein can be used to prepare protein molecular markers for judging the prognosis of breast cancer patients. Postoperative monitoring and sequential treatment also have important guiding significance.
Owner:FUJIAN NORMAL UNIV
Method for breast cancer recurrence prediction under endocrine treatment
ActiveUS20170067118A1Reliable predictionMicrobiological testing/measurementMaterial analysisDisease outcomeEndocrine therapy
The present invention relates to methods, kits and systems for the prognosis of the disease outcome of breast cancer, said method comprising:(a) determining in a tumor sample from said patient the RNA expression levels of at least 2 of the following 9 genes: UBE2C, BIRC5, RACGAP1, DHCR7, STC2, AZGP1, RBBP8, IL6ST, and MGP(b) mathematically combining expression level values for the genes of the said set which values were determined in the tumor sample to yield a combined score, wherein said combined score is indicative of a prognosis of said patient; and kits and systems for performing said method.
Owner:SIVIDON DIAGNOSTICS
Assay for the detection of recurrence in breast cancer using the novel tumor suppressor dear1
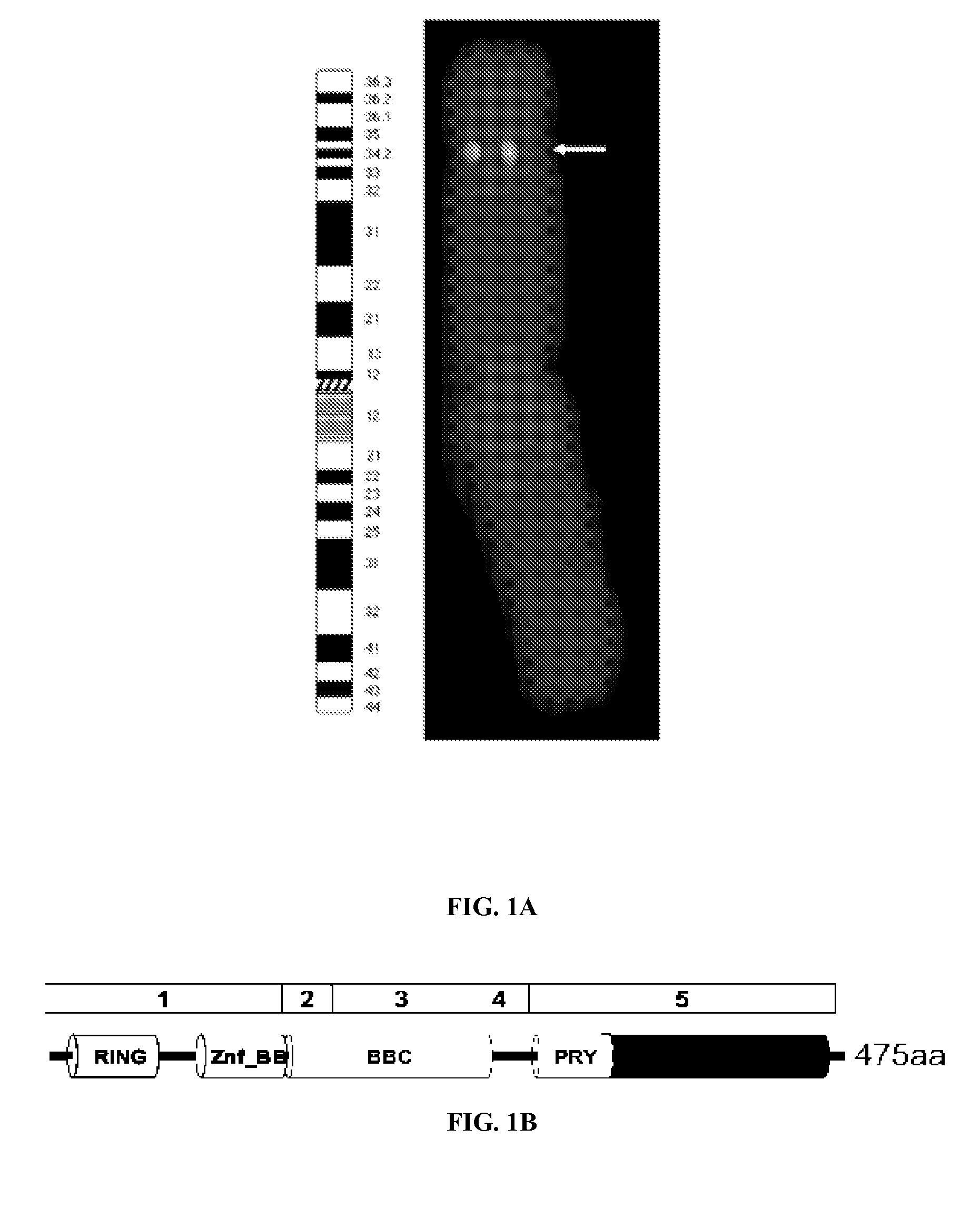
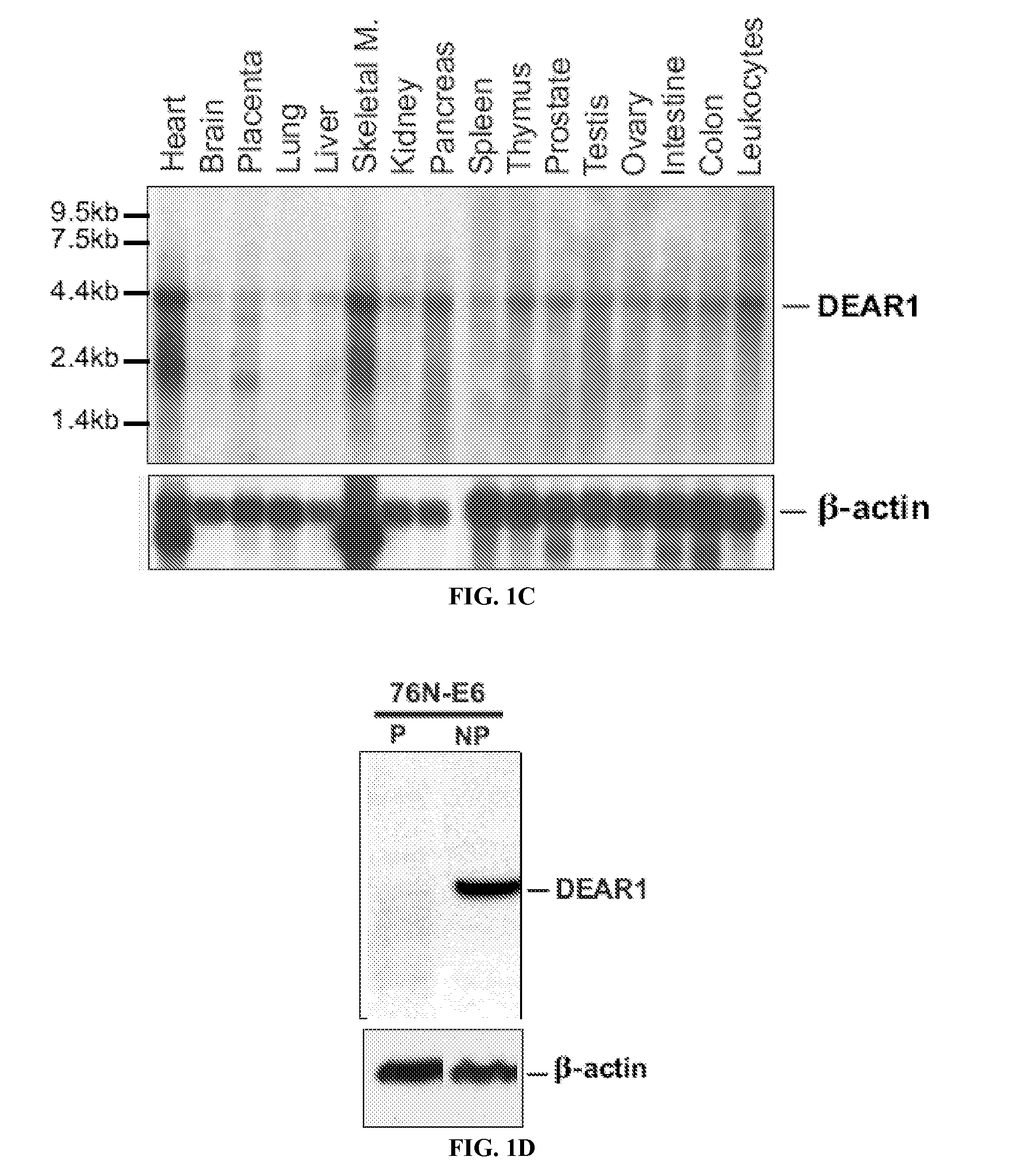
InactiveUS20120058901A1High riskLow chanceElectrolysis componentsChemiluminescene/bioluminescenceAbnormal tissue growthSuppressor
Certain aspects of the present invention relate to new diagnostic and prognostic methods involving DEAR1, a gene is located on the short arm of human chromosome 1. Specifically, analysis of expression or structure of this gene for prognosis or recurrence risk assessment is disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of predicting breast cancer prognosis
InactiveUS20160222463A1Microbiological testing/measurementDisease diagnosisIntergenic SequenceOncology
The present invention relates to biomarkers associated with breast cancer prognosis. These biomarkers include coding transcripts and their expression products, as well as non-coding transcripts, and are useful for predicting the likelihood of breast cancer recurrence in a breast cancer patient, The present invention also relates to a novel method of identifying intergenic sequences that correlate with a clinical outcome.
Owner:GENOMIC HEALTH INC
Predicting breast cancer recurrence
InactiveUS20150203921A1Improved prognosisSimpler and accurate applicationMicrobiological testing/measurementLibrary screeningOncologyBreast cancer recurrence
Provided are methods of determining risk of cancer recurrence in a subject afflicted with breast cancer. Also provided are methods of determining responsiveness to treatment of a subject afflicted with breast cancer. Additionally provided are methods of treating a subject afflicted with breast cancer.
Owner:BIOTHERANOSTICS +1
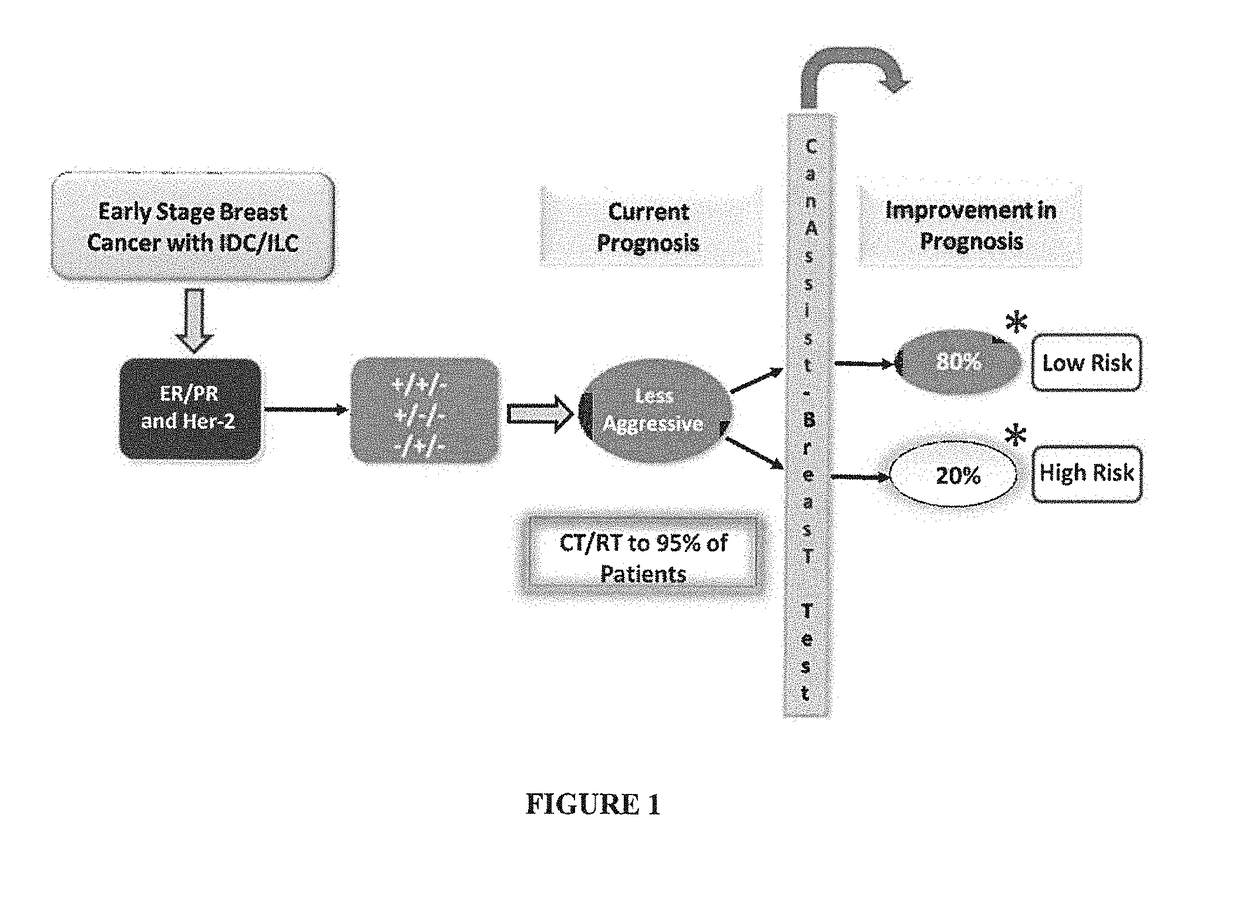
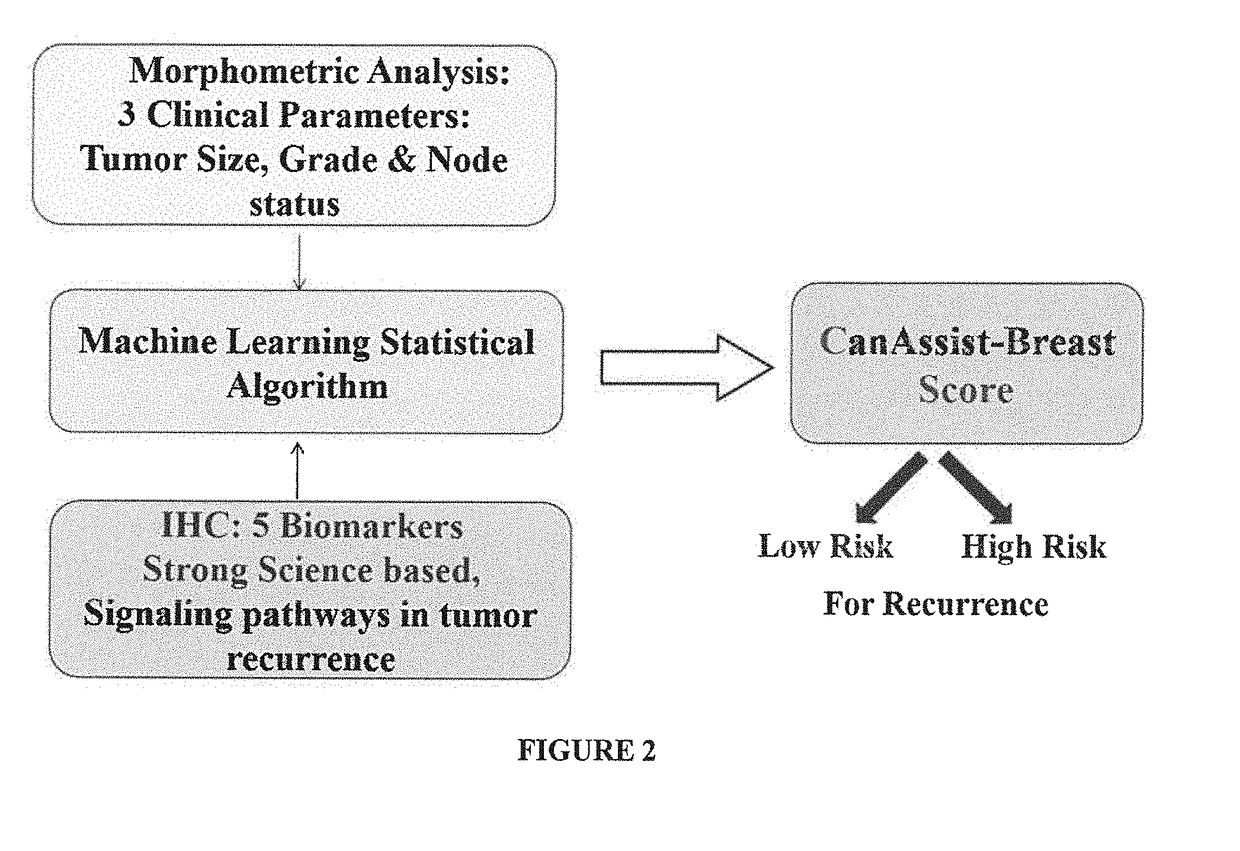
Method of prognosing and predicting breast cancer recurrence, markers employed therein and kit thereof
The present disclosure relates to a method of prognosing and predicting breast cancer recurrence in patients, More particularly, the present disclosure relates to a method of prognosing which stratifies early stage ER+ / PR+ and Her2− breast cancer patients with invasive ductal / lobular carcinoma of the breast into low risk or high risk for breast cancer recurrence by employing an IHC based assay which assesses or measures expression of a combination of 5 biomarkers and by employing a histopathological analysis which assesses 3 clinical prognostic parameters. The present disclosure also relates to a combination of 5 biological markers and 3 clinical prognostics markers employed in the method for prognosis of breast cancer, a kit / test comprising the antibodies against said markers for said prognosis and an IHC based assay system comprising said markers.
Owner:ONCOSTEM PTE LTD
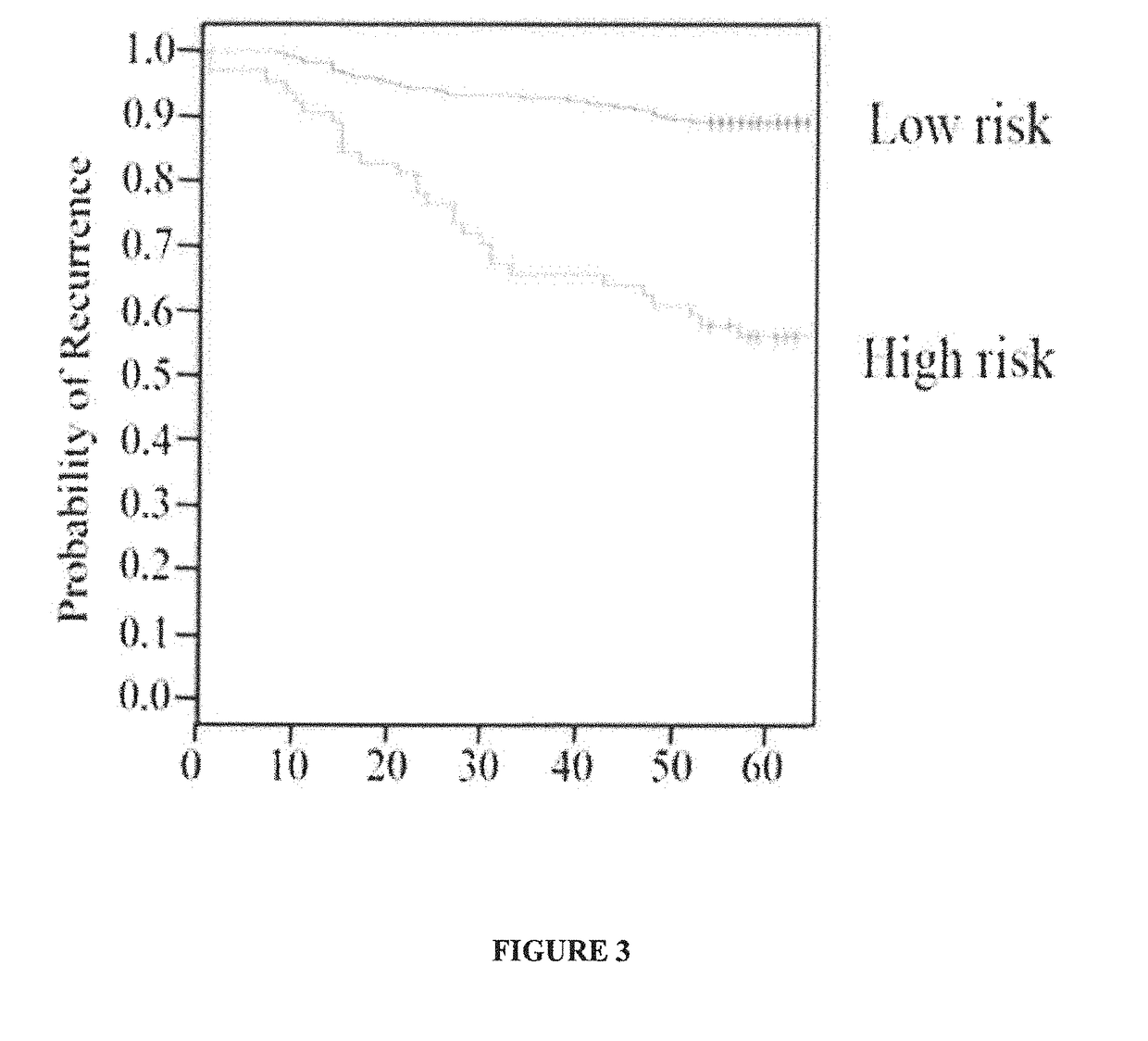
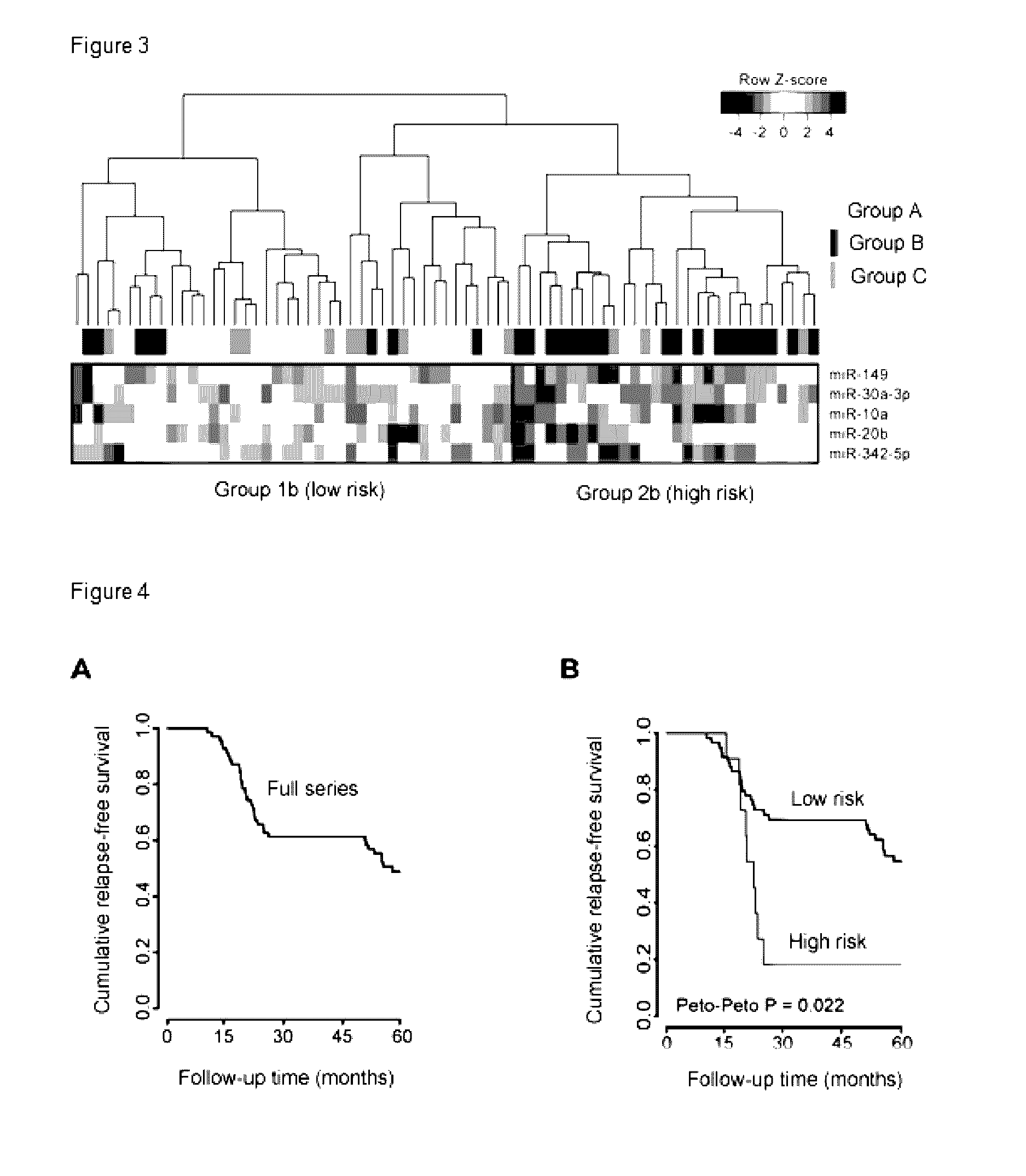
Microrna signature as an indicator of the risk of early recurrence in patients with breast cancer
The invention relates to the field of oncology and cancer treatment. The invention relates to methods for predicting the risk of recurrence of breast tumors using the expression signature of particular miRNAs. Specifically, the invention relates to a method for determining the risk of recurrence of breast cancer which comprises measuring the expression levels of at least one miRNA selected from the group consisting of miR-149-5p (SEQ ID NO: 1), miR-10a-5p, (SEQ ID NO: 2), miR-20b-5p, (SEQ ID NO: 3), miR-30a-3p (SEQ ID NO: 4), and miR-342-5p, (SEQ ID NO: 5) in a sample of the tumor, wherein a change in the expression level of at least one miRNA in the tumor with respect to the expression level in a control sample is indicative of a high risk of recurrence of the tumor. The invention also relates to tools and kits for carrying out the method of the invention.
Owner:FUNDACION PUBLICA ANDALUZA PARA LA INVESTIGACION DE MALAGA & BIOMEDICINA Y SALUD FIMABIS +2
Assessing risk of breast cancer recurrence
ActiveUS10489904B2Adverse side-effects can be avoidedReduce riskImage enhancementImage analysisData setRisk classification
The subject disclosure presents systems and computer-implemented methods for assessing a risk of cancer recurrence in a patient based on a holistic integration of large amounts of prognostic information for said patient into a single comparative prognostic dataset. A risk classification system may be trained using the large amounts of information from a cohort of training slides from several patients, along with survival data for said patients. For example, a machine-learning-based binary classifier in the risk classification system may be trained using a set of granular image features computed from a plurality of slides corresponding to several cancer patients whose survival information is known and input into the system. The trained classifier may be used to classify image features from one or more test patients into a low-risk or high-risk group.
Owner:VENTANA MEDICAL SYST INC +1
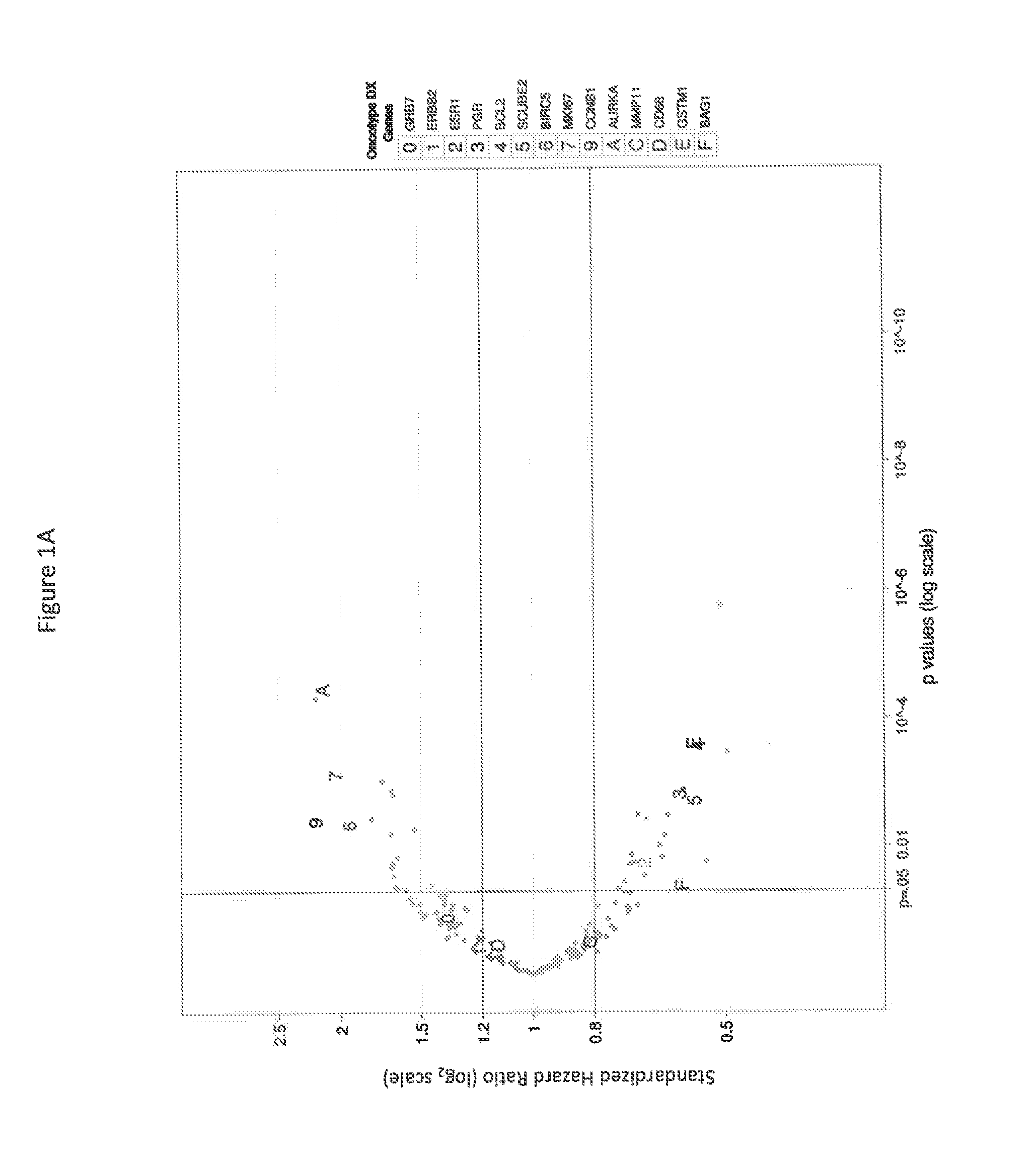
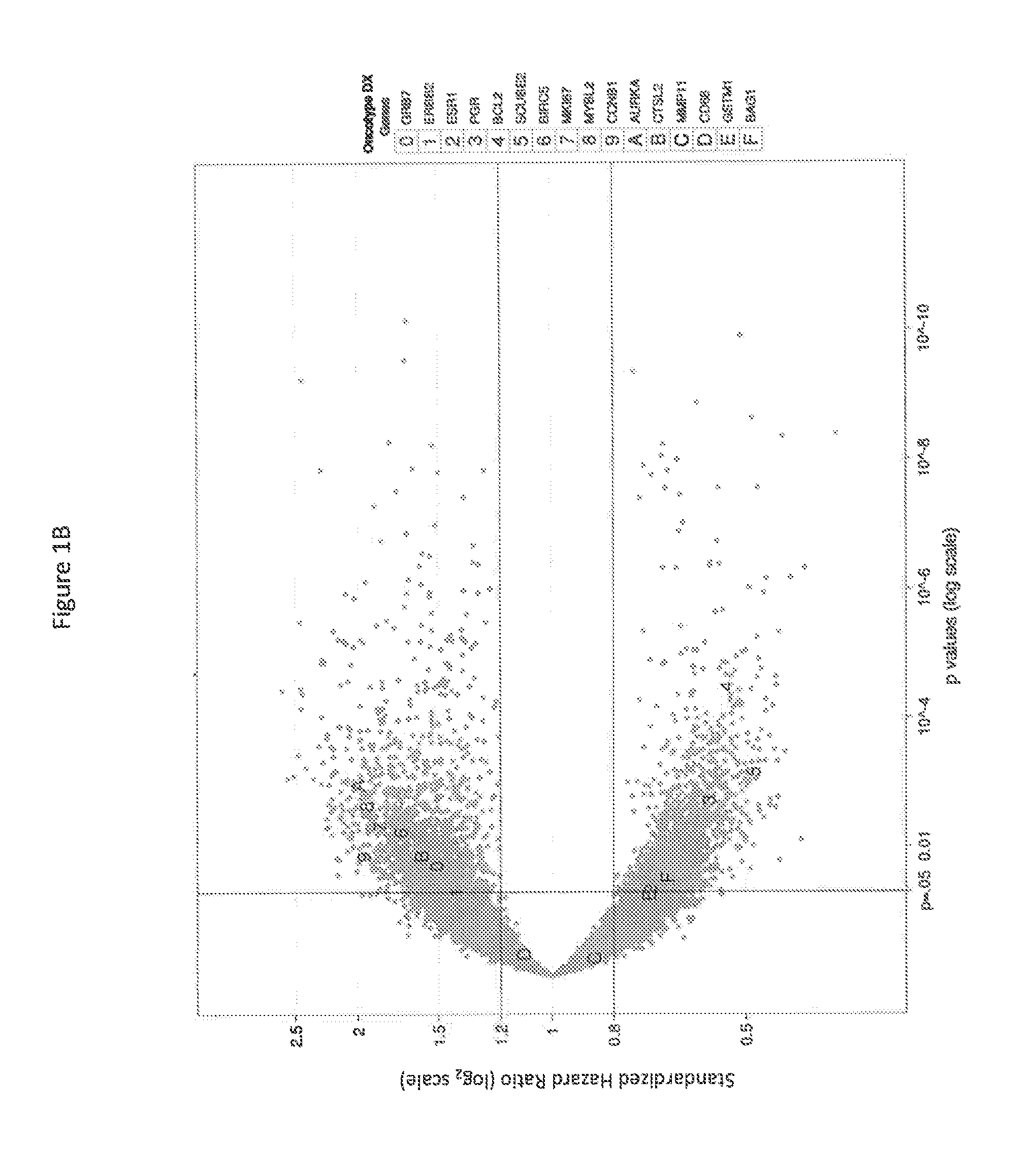
Transcriptomic profiling for prognosis of breast cancer
PendingCN113557310AMechanical/radiation/invasive therapiesHealth-index calculationEndocrine therapyBiologic marker
Methods, systems, and kits for the diagnosis, prognosis, and treatment of breast cancer in a subject are disclosed. The invention also provides biomarkers and clinically useful classifiers for identifying subjects at low risk of breast cancer recurrence who may be spared from adjuvant chemotherapy and endocrine therapy. Further disclosed herein, in certain instances, are probe sets for use in detecting such biomarkers for determining the risk of breast cancer recurrence in a subject. Methods of beating breast cancer based on expression profiling to determine the risk of breast cancer recurrence are also provided.
Owner:PFS GENOMICS INC +1
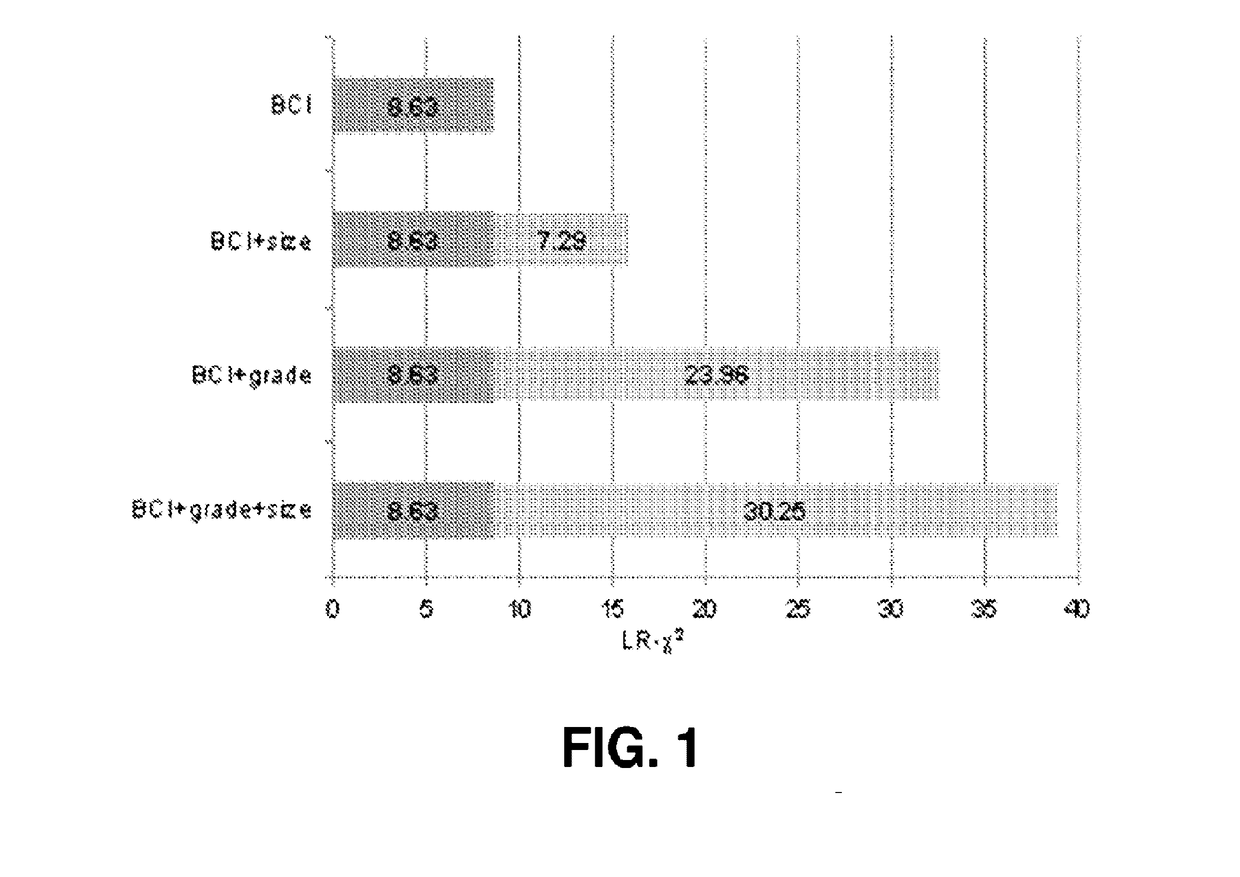
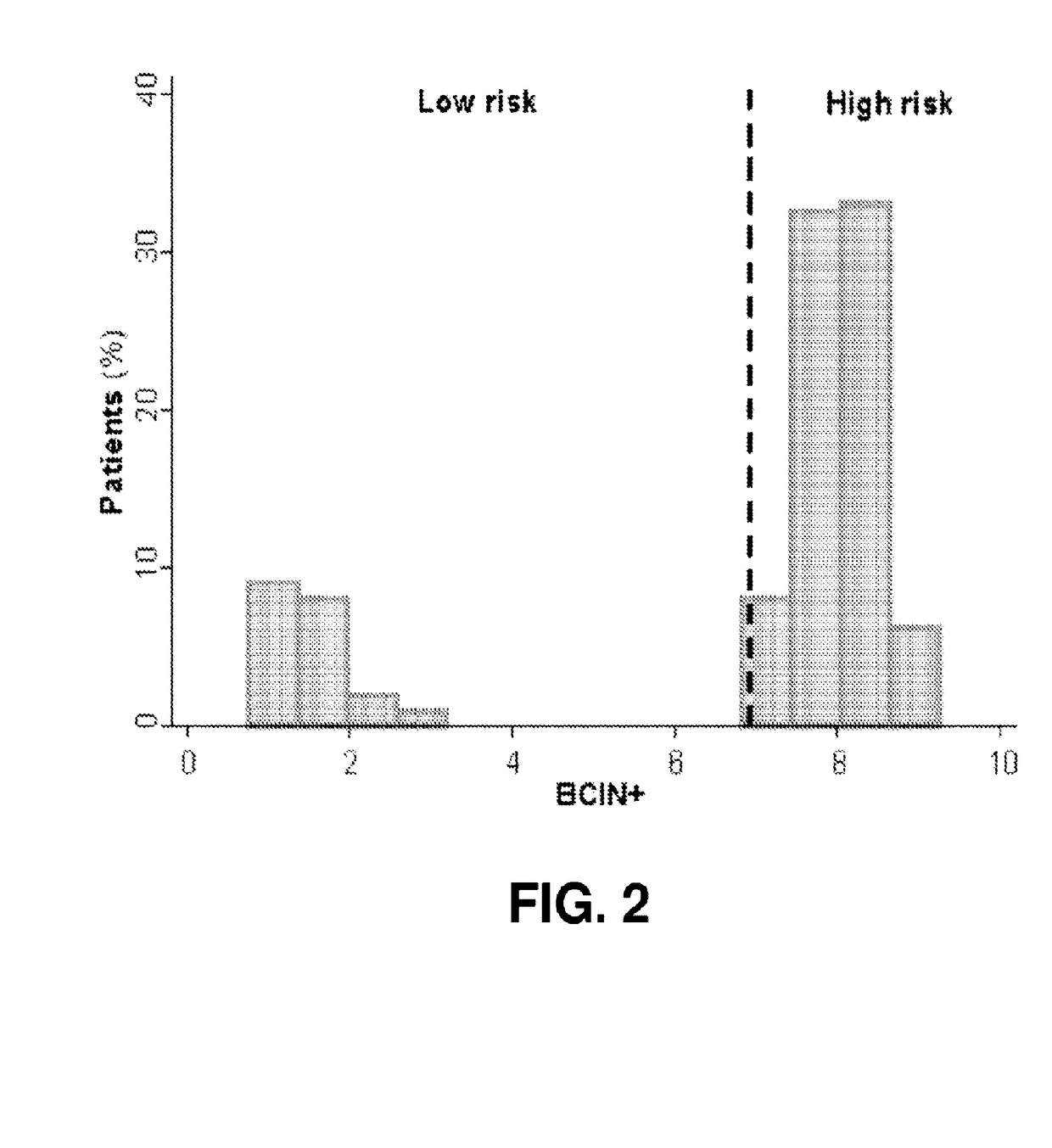
Integration of tumor characteristics with breast cancer index
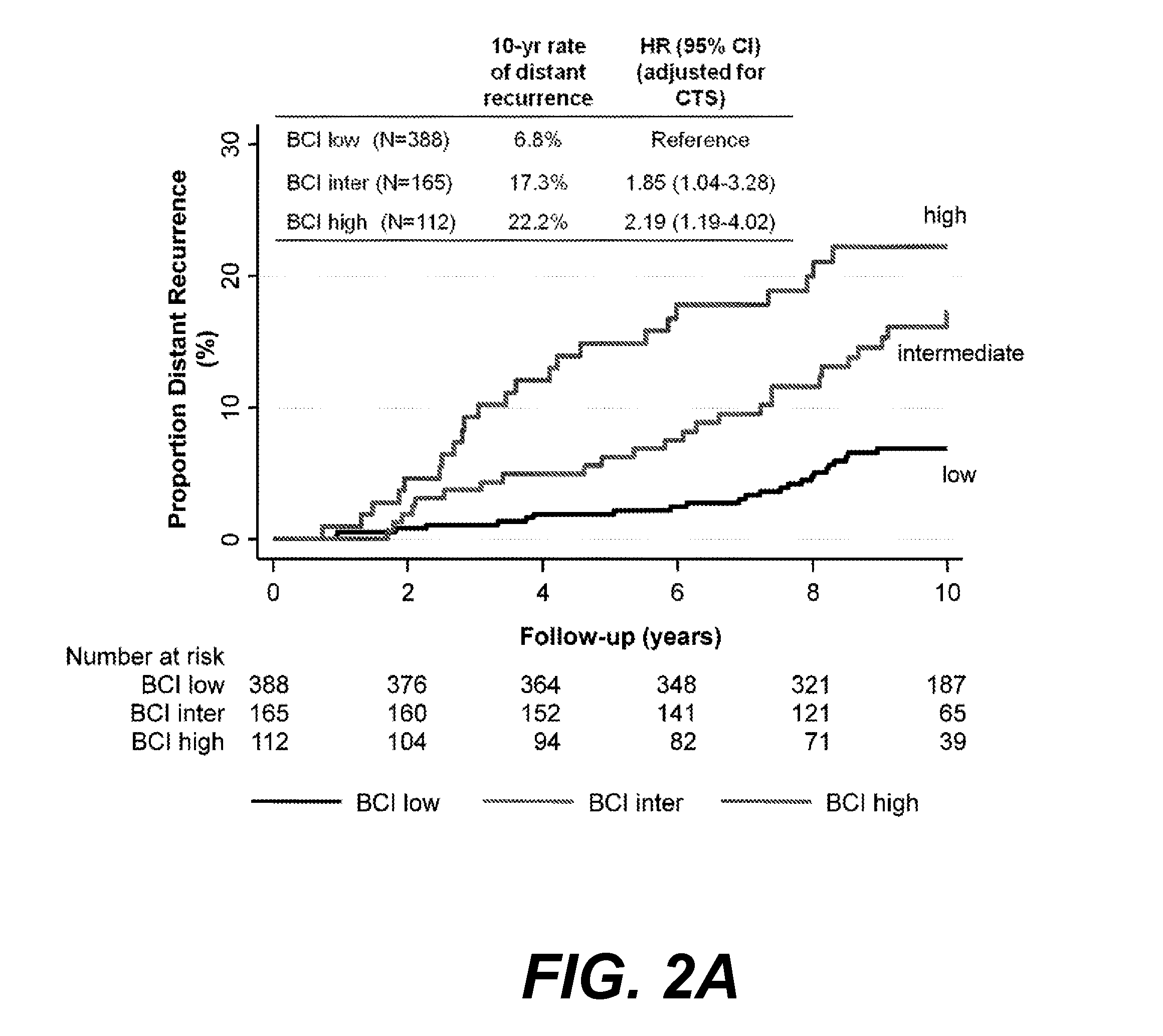
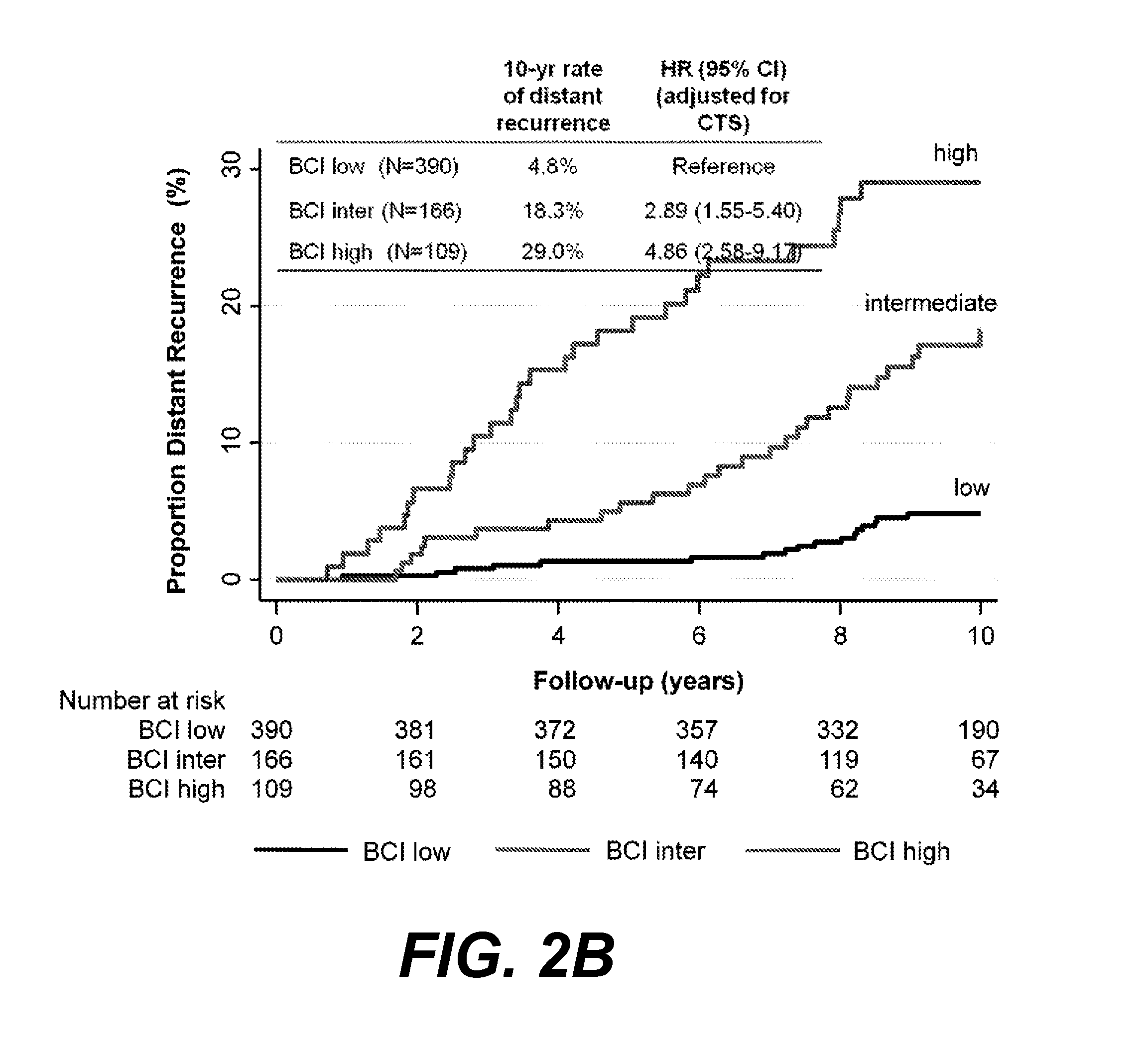
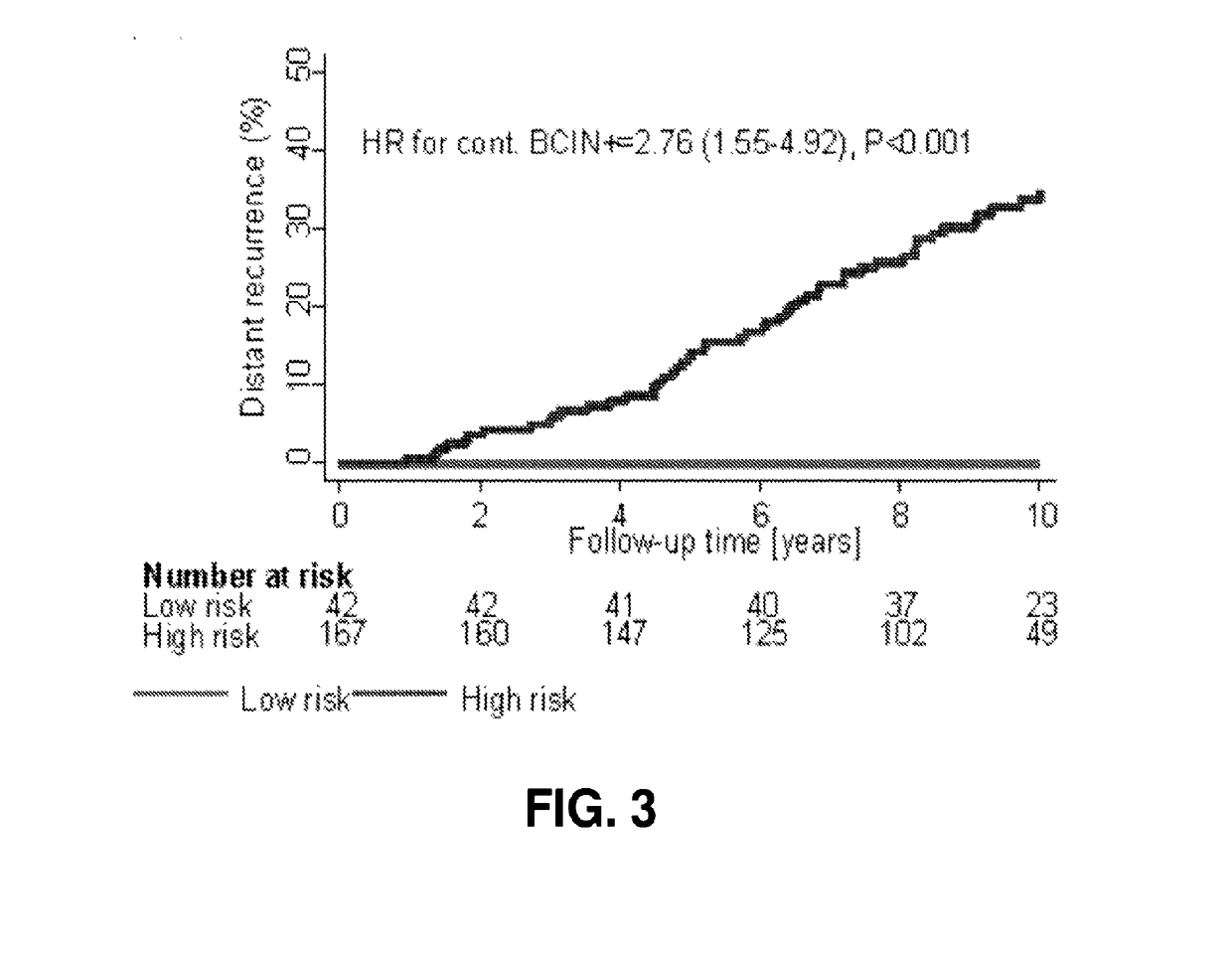
ActiveUS20170137891A1Reduce riskHigh riskOrganic active ingredientsMicrobiological testing/measurementOncologyCvd risk
Methods of determining risk of recurrence of a breast cancer of a subject are provided. Also provided are methods of predicting responsiveness to a therapy of a breast cancer of a subject. Additionally, methods of recommending treatment for a subject that has breast cancer are provided. Further provided are methods of treating a subject that has breast cancer. Systems for performing described methods are also provided.
Owner:BIOTHERANOSTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com