Inhalation solutions
An inhaler, co-solvent technology for applications in the treatment of asthma, COPD (chronic obstructive pulmonary disease) and other breathing disorders, which can solve problems such as uninstructed doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
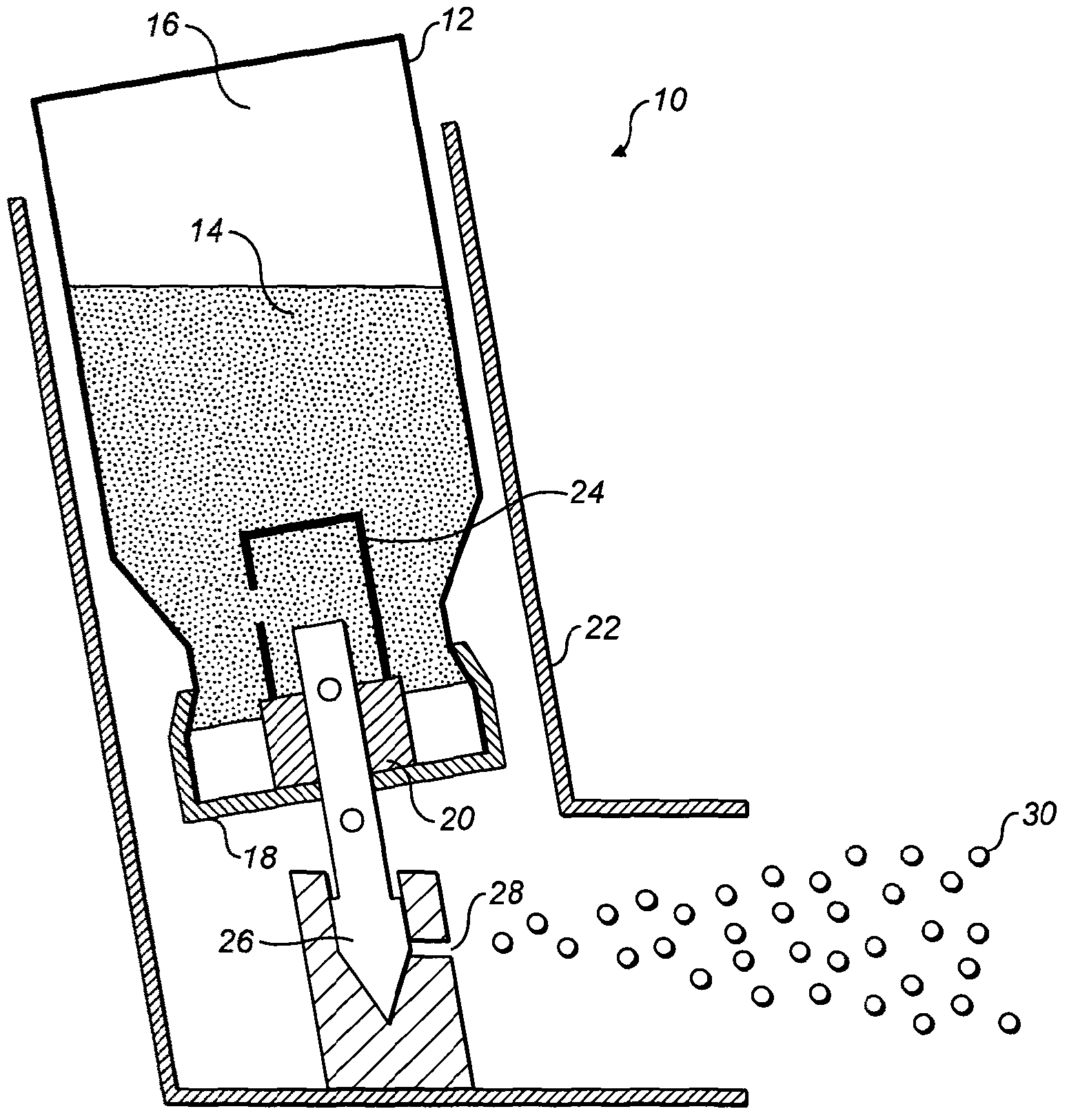
[0031] As discussed herein, the inventors have surprisingly found that administration of a pressurized aerosol inhalable formulation comprising an active agent in solution via a low-porosity drive results in the desired FPD of the active agent particles / aerosol particles .
[0032]The low bore driver advantageously has a bore diameter of 0.2mm-0.4mm. Preferably, said diameter is 0.2mm to 0.33mm, more preferably 0.28mm to 0.33mm.
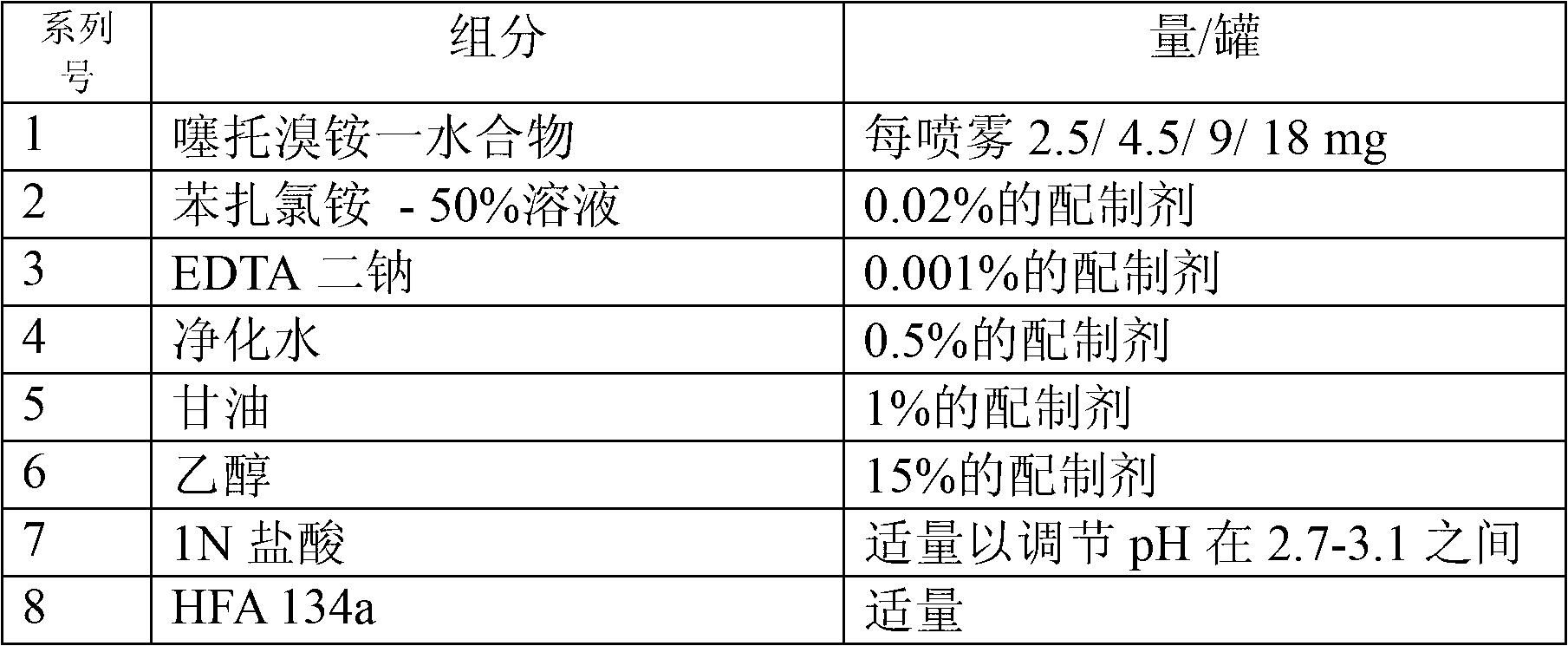
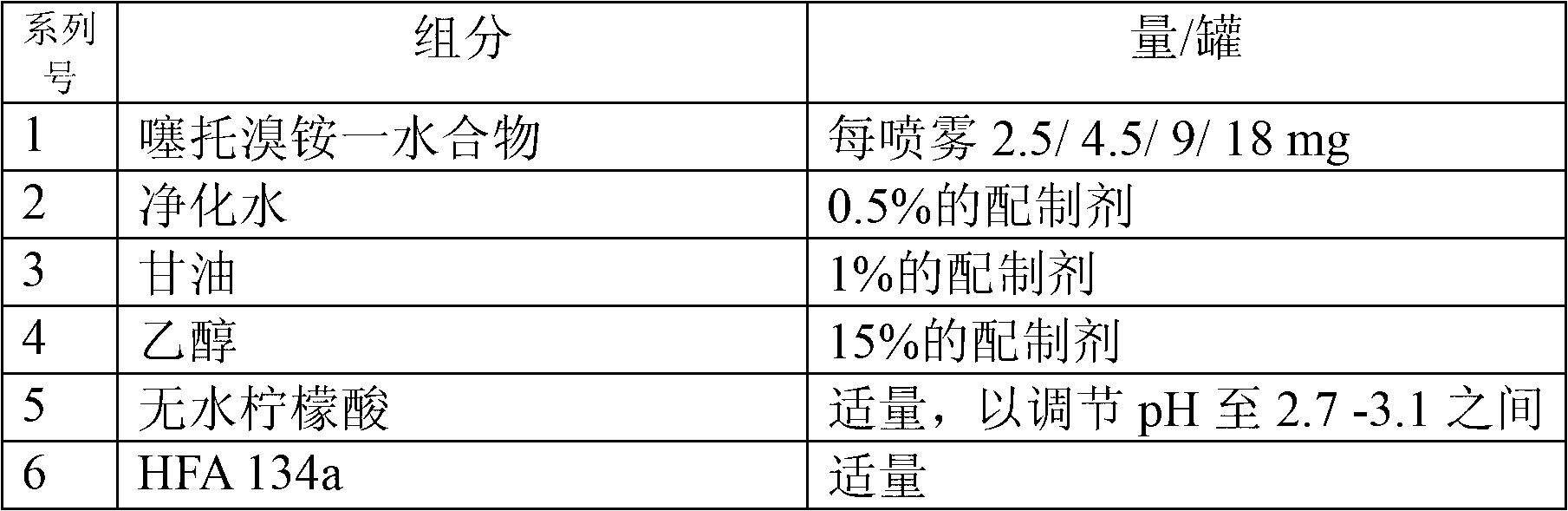
[0033] The inhalable solution according to the present invention may include one or more anticholinergic drugs, including tiotropium, ipratropium, oxitropium, aclidinium or their pharmaceutically acceptable salts and solvates , tautomers, derivatives, optical enantiomers, isomers, hydrates, prodrugs or polymorphs thereof.
[0034] Preferred salts are bromide salts, especially bromide salts of tiotropium, ipratropium, oxitropium and aclidinium. According to the invention, in a particularly preferred embodiment, the inhalation solution may comprise ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com