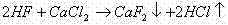Method for recovering fluoride ions in freon by-product mixed acid
A mixed acid and freon technology, applied in the direction of calcium/strontium/barium fluoride, calcium/strontium/barium halide, chlorine/hydrogen chloride, etc., to achieve the effect of improving the quality of hydrochloric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 1300 Kg of calcium chloride aqueous solution with a concentration of 40% into the reactor, heat up to 70°C under stirring; control the dropping rate for about 1 hour, add 1000 Kg of 1% hydrofluoric acid and Freon by-product mixed acid was added dropwise to the above-mentioned calcium chloride solution, and the hydrogen chloride gas produced was absorbed with 507 Kg of 3% dilute hydrochloric acid to obtain 725 Kg of 30% industrial hydrochloric acid with a hydrofluoric acid concentration of 0.021%. Continue to heat up until the temperature of the kettle reaches 120°C, and the distillate is condensed to obtain 827Kg of 3% dilute hydrochloric acid. Cool the kettle liquid to about 70°C, centrifugally filter and separate the calcium fluoride generated by the reaction, rinse the filter cake with 25Kg of water, and dry it to obtain 23Kg of calcium fluoride with a water content of 15%, and the recovery rate of fluoride ions is 98.48%. The filtrate is pumped into the reaction...
Embodiment 2
[0027] Add 2200 Kg of calcium chloride aqueous solution with a concentration of 25% into the reactor, heat up to 60°C under stirring; control the dropping rate for about 2 hours, add 1000 Kg of 5% hydrofluoric acid and Freon by-product mixed acid was added dropwise to the above-mentioned calcium chloride solution, and the hydrogen chloride gas produced was absorbed with 680 Kg of 4% dilute hydrochloric acid to obtain 971 Kg of 30% industrial hydrochloric acid with a hydrofluoric acid concentration of 0.019%. Continue to heat up until the kettle temperature reaches 108°C, and the distillate is condensed to obtain 745Kg of 4% dilute hydrochloric acid. Cool the kettle liquid to about 60°C, centrifugally filter and separate the calcium fluoride generated by the reaction, rinse the filter cake with 150Kg water, and dry it to obtain 114Kg of calcium fluoride with a water content of 15%, and the recovery rate of fluoride ions is 99.63%. The filtrate is pumped into the reactor, and 13...
Embodiment 3
[0029] Add 2500 Kg of calcium chloride aqueous solution with a concentration of 20% into the reactor, and heat up to about 50°C under stirring; control the dropping rate for about 3 hours, and add 500 Kg of 10% hydrofluoric acid and a total acid concentration of 30% The Freon by-product mixed acid is added dropwise in the above-mentioned calcium chloride solution, and the hydrogen chloride gas produced is absorbed with 446 Kg of 5% dilute hydrochloric acid to obtain 637Kg of 30% industrial hydrochloric acid with a hydrofluoric acid concentration of 0.025%. Continue to heat up until the temperature of the kettle reaches 105°C, and the distillate is condensed to obtain 345Kg of dilute hydrochloric acid with about 5%. Cool the kettle liquid to about 50°C, centrifugally filter and separate the calcium fluoride generated by the reaction, rinse the filter cake with 150Kg water, and dry it to obtain 114Kg of calcium fluoride with a water content of 15%, and the recovery rate of fluori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com