Polyferose controlled-release pellet and preparation method thereof
A technology for slow-release pellets and polysaccharide iron, applied in the field of medicine, can solve the problems of rapid increase in plasma concentration, etc., and achieve the effects of avoiding the peak-to-valley phenomenon of blood drug concentration, effective absorption, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
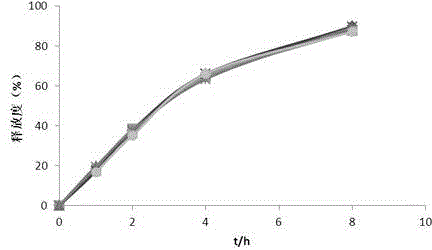
Embodiment 1
[0035] The contents of the principal ingredient and auxiliary materials in the polysaccharide iron sustained-release preparation are as follows:
[0036] The composition of the pill core containing medicine is: 350 parts of polysaccharide iron, 35 parts of hypromellose E5 and 160 parts of blank ball core;
[0037] The composition of the delayed release film coat was: 29.17 parts ethylcellulose, 2.92 parts hypromellose E5 and 2.92 parts castor oil.
[0038] The preparation method of the polysaccharide iron sustained-release pellets comprises the following steps:
[0039] (1) Preparation of drug-containing pill cores: take the polysaccharide iron, blank pellet cores and hypromellose E5 in the ratio described above, add hypromellose E5 into an appropriate amount of hot water to disperse, cool to dissolve, and prepare Containing the adhesive of 3% hypromellose E5, adding the blank ball core in the centrifugal granulator, spraying the adhesive while carrying out the polysaccharide...
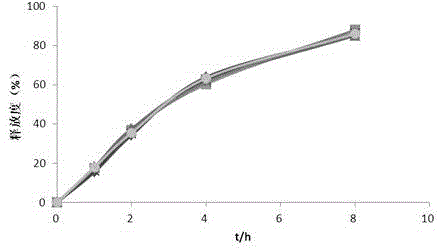
Embodiment 2
[0054] The contents of the principal ingredient and auxiliary materials in the polysaccharide iron sustained-release preparation are as follows:
[0055] The composition of the pill core containing medicine is: 350 parts of polysaccharide iron, 35 parts of hypromellose E5 and 148 parts of blank ball core;
[0056] The composition of the sustained release film coat was: 35.83 parts ethyl cellulose, 3.58 parts polyethylene glycol 1500 and 3.58 parts castor oil.
[0057] The preparation method of the above-mentioned polysaccharide iron sustained-release pellets comprises the following steps:
[0058] (1) Preparation of drug-containing pill cores: take the polysaccharide iron, blank pellet cores and hypromellose E5 in the ratio described above, add hypromellose E5 into an appropriate amount of hot water to disperse, cool to dissolve, and prepare Contain the binder of 3% hypromellose E5, add the blank pellet core in the centrifugal granulator, spray the binder while carrying out t...
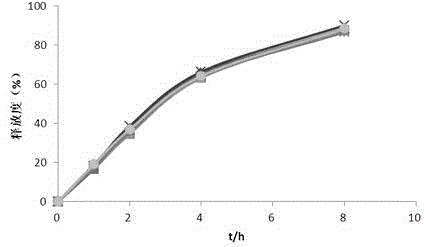
Embodiment 3
[0063] The contents of the principal ingredient and auxiliary materials in the polysaccharide iron sustained-release preparation are as follows:
[0064] The composition of the pill core is: 350 parts of polysaccharide iron, 35 parts of hypromellose E5 and 167 parts of blank pill core;
[0065] The sustained-release film coating consisted of: 23.33 parts of cellulose acetate, 2.33 parts of polyethylene glycol 1500 and 2.33 parts of castor oil.
[0066] The preparation method of the above-mentioned polysaccharide iron sustained-release pellets comprises the following steps:
[0067] (1) Preparation of drug-containing pill cores: take the polysaccharide iron, blank pellet cores and hypromellose E5 in the ratio described above, add hypromellose E5 into an appropriate amount of hot water to disperse, cool to dissolve, and prepare Contain the binder of 3% hypromellose E5, add the blank pellet core in the centrifugal granulator, spray the binder while carrying out the polysaccharid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com