Method for preparing acetic acid through catalytic carbonylation reaction
A carbonylation reaction and a reaction technology, applied in the field of preparing acetic acid by catalytic carbonylation reaction, can solve the problems of short oxidation time of product potassium permanganate, poor stability of rhodium catalyst, low reaction activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
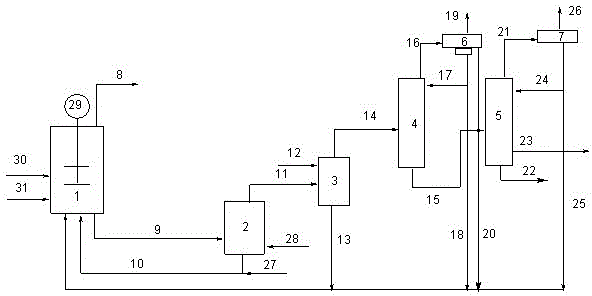
[0045] Ruthenium complex Li + / H + [Ru(CO) 3 I 3 ] - Preparation: add ruthenium acetate or ruthenium iodide to acetic acid solution, add lithium iodide and water, HI, introduce CO, react at 3.0MPa, 190°C for 2h, and separate the ruthenium complex Li + / H + [Ru(CO) 3 I 3 ] - , IR v (CO): about 2106.7cm -1 , about 2039.0cm -1 , NMR spectrum, carbonyl peak position: 13 CNMR (100 MHz, CDCl 3 ): δ about 186.3ppm, through Xevo G2 QTof mass spectrometry, ESI / negative: 566.6020.
[0046]Add rhodium acetate, methyl iodide, distilled water, methyl acetate and lithium iodide once in the zirconium material autoclave that pressure gauge is housed in 200ml, make the rhodium concentration in the reaction system be 700ppm, methyl iodide 12wt%, distilled water 6wt%, Methyl acetate is 20wt%, lithium iodide is 6wt%, and the rest is acetic acid solvent. Keep the reaction temperature at 190°C and the reaction pressure at 3.0MPa. The average STY of the acetic acid reacted was 7.5 mo...
Embodiment 2
[0048] Ruthenium complex Li + / H + [Ru(CO)I 3 ] - , Li + / H + [Ru(CO) 2 I 3 ] - , Li + / H + [Ru(CO) 3 I 3 ] - Preparation: add ruthenium acetate or ruthenium iodide to acetic acid solution, add lithium iodide, water, HI, introduce CO, react at 2.0MPa, 190°C for 1h, and further separate and obtain the ruthenium complex Li + / H + [Ru(CO)I 3 ] - , Li + / H + [Ru(CO) 2 I 3 ] - , Li + / H + [Ru(CO) 3 I 3 ] - , Xevo G2 QTof mass spectrum, ESI / negative: 566.6020, 538.6073, 510.6120.
[0049] Add rhodium acetate, methyl iodide, distilled water, methyl acetate and lithium iodide once in the zirconium material autoclave that pressure gauge is housed in 200ml, make the rhodium concentration in the reaction system be 700ppm, methyl iodide 12wt%, distilled water 6wt%, Methyl acetate is 20wt%, lithium iodide is 6wt%, and the rest is acetic acid solvent. Keep the reaction temperature at 190°C and the reaction pressure at 3.0MPa. The average STY of the acetic...
Embodiment 3
[0051] Ruthenium complex [Ru(CO) 4 I 2 ] Preparation: put Ru 3 (CO) 12 and I 2 Dissolved in n-hexane, cooled to -40°C through a dry ice-acetone bath, then gradually warmed to room temperature, removed the solvent and dried in vacuum to obtain a brown solid, which was obtained by infrared analysis as [Ru(CO) 4 I 2 ] and [Ru(CO) 3 I 2 ] 2 mixture, the mixture was dissolved in CH 2 Cl 2 In the airtight container, 1.0 MPa of CO gas was introduced, and then heated to 40°C. After the reaction, the solvent CH was removed. 2 Cl 2 , to obtain a yellow solid as [Ru(CO) 4 I 2 ], IR v(CO) / cm -1 (CH 2 Cl 2 ): 2161, 2106, 2097, 2068.
[0052] Add rhodium acetate, methyl iodide, distilled water, methyl acetate and lithium iodide once in the zirconium material autoclave that pressure gauge is housed in 200ml, make the rhodium concentration in the reaction system be 700ppm, methyl iodide 12wt%, distilled water 6wt%, Methyl acetate is 20wt%, lithium iodide is 6wt%, and the res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com