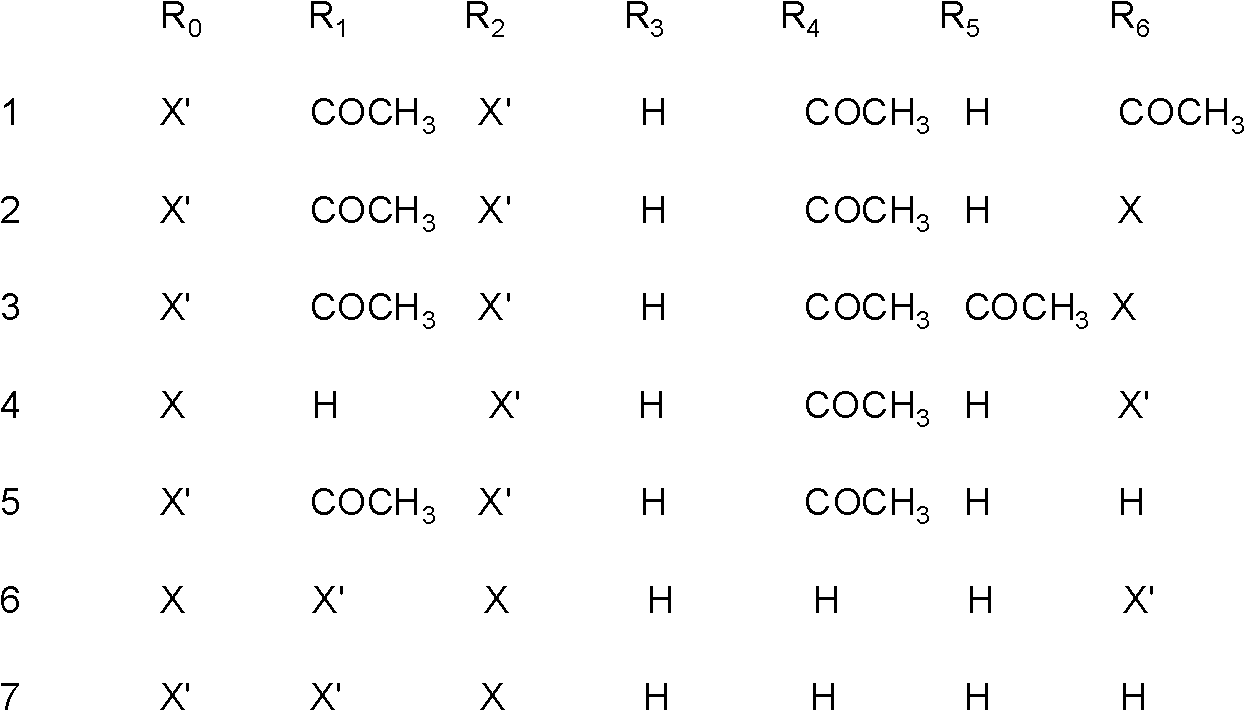Tartary buckwheat phenylpropanoid glycoside compound and preparation method and application thereof
A technology of buckwheat phenylpropanoid glycosides and compounds is applied in the field of preparation of such tartary buckwheat phenylpropanoside compounds, and can solve the problems such as no reports on antitumor research on chemical constituents of tartary buckwheat roots.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: Preparation of phenylpropanoid glycosides 1-7
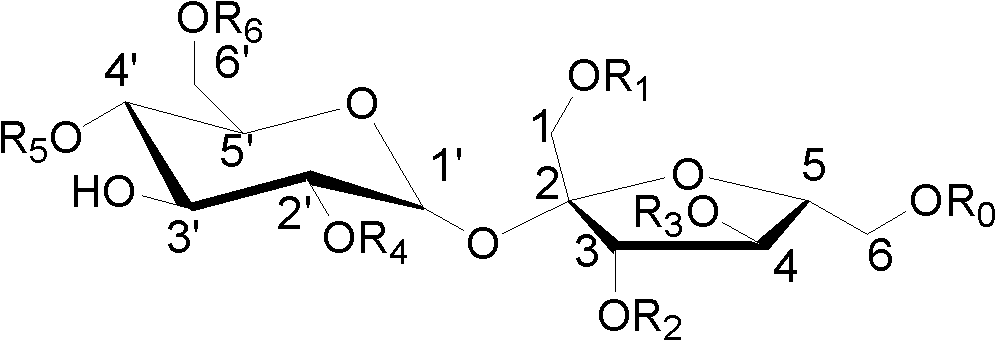
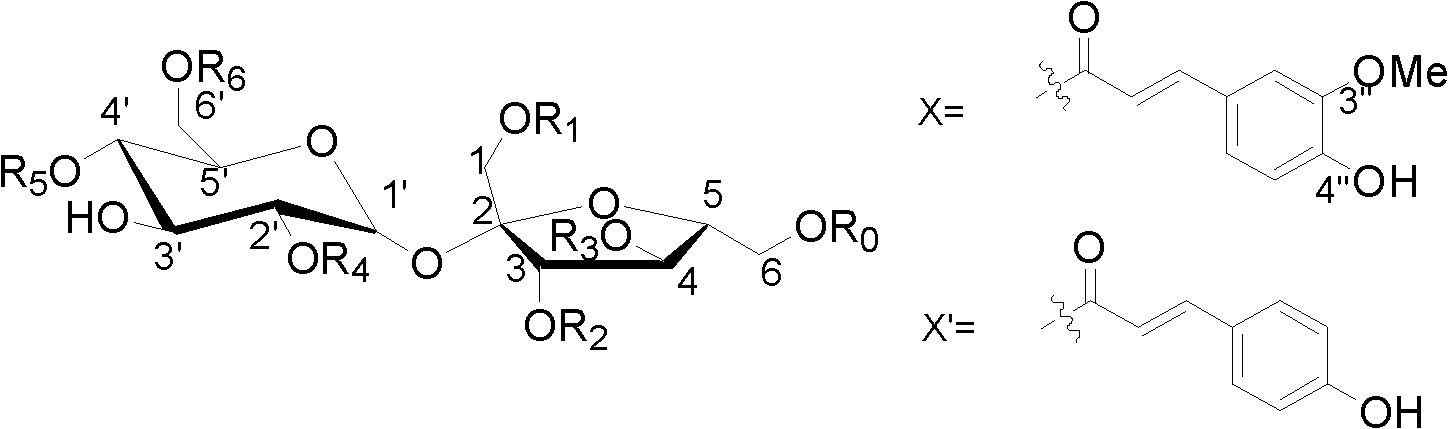
[0014] Weigh 1000g of tartary buckwheat root, and heat extract 3 times with 80% ethanol, each time the amount of 80% ethanol is 9000g, each extraction time is 2 hours, combine the extracts and concentrate to dryness to obtain the tartary buckwheat root extract 80g, then suspended with 80g of water, degreased with dichloromethane, extracted with ethyl acetate, loaded the ethyl acetate part into silica gel column chromatography, and eluted with dichloromethane-methanol gradient (50:1, 30: 1, 15:1, 5:1, 3:1), combined to obtain three fragments (I-III). Fragment I was separated by repeated silica gel column chromatography, and dichloromethane-methanol (20:1, 10:1) was eluted to obtain two eluted fragments (I 1 , I 2 ). Fragment I 1 Compound 1 and compound 2 were obtained by loading sephadex LH-20 and eluting with 80% methanol-water. Fragment I 2 Compound 3 was obtained by sephadex LH-20 chromatography elute...
Embodiment 2
[0030] Example 2: Inhibitory effect of compounds provided by the invention on the growth of related tumor cells
[0031] (1) Sample preparation: after dissolving 0.5mg of each sample of compound 1-7 with 50ul of DMSO (Merck), the concentration is 10mg / ml, take 10ul and add PBS(-)90ul to make a solution of 1000μg / ml or a uniform suspension The solution was added to the 96-well plate as the first concentration, and the subsequent concentrations were 10-fold dilutions of the previous concentration, and the final concentrations were 100, 10, 1, 0.1, 0.01, and 0.001 μg / ml.
[0032] (2) Cell lines: A549 (human lung cancer cells), HCT116 (human colon cancer cells), ZR-75-30 (human breast cancer cells), HL-60 (human leukemia cells), all of the above cell lines were provided by the Second Military Doctor Cryopreservation and passage in the Department of Pharmacognosy, School of Pharmacy, University.
[0033] (3) Culture medium: DMEM+10-15% FBS+double antibody.
[0034] (4) Test metho...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap