Preparation of intumescent type flame retardant synergist on basis of magnesium oxide flue gas desulfurization residue
A technology of flame retardant synergist and desulfurization waste residue, applied in the field of intumescent flame retardant synergist, can solve the problems of unstable chemical properties, easy moisture absorption, low content, etc., to achieve high synergy efficiency, high added value, raw materials low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
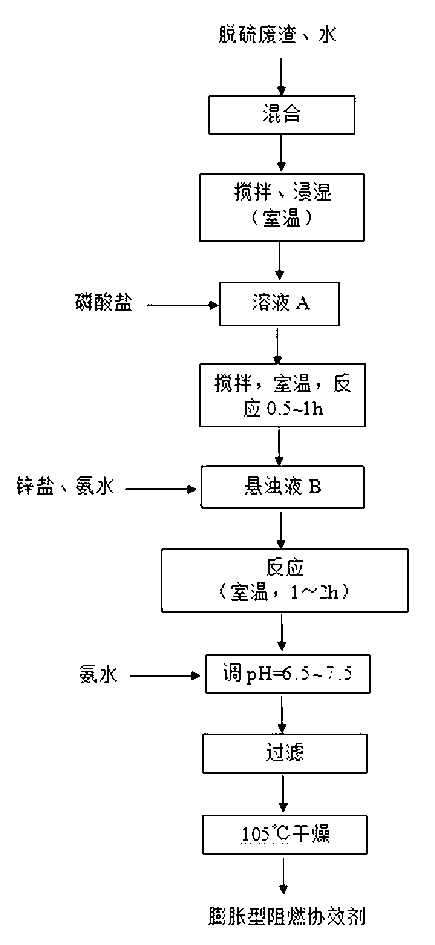
[0029] For the preparation process, see the attached figure 1
[0030] (1) Add 100mL of distilled water into a beaker, add 3g of dry magnesium oxide desulfurization waste residue, stir evenly at room temperature until the waste residue is completely wetted, and prepare a suspension A with a mass concentration of 3%;
[0031] (2) Add 0.01mol sodium dihydrogen phosphate to the above suspension A to obtain suspension B, stir and absorb at room temperature for 0.5 hours, then add 0.01mol ammonia water and 0.011mol zinc chloride to the above suspension B at the same time. The reaction was stirred at room temperature for 1 hour to obtain a suspension C;
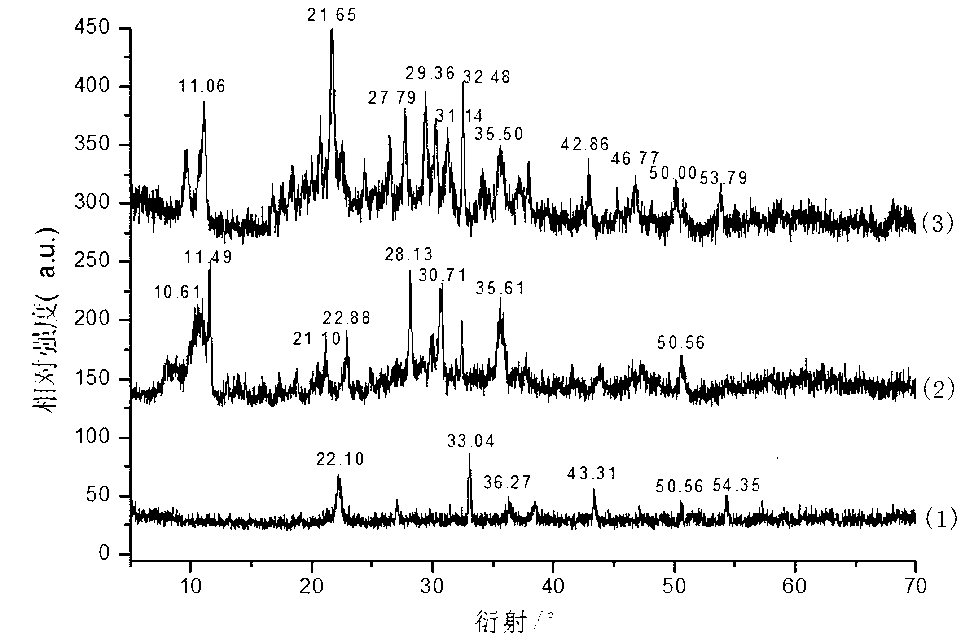
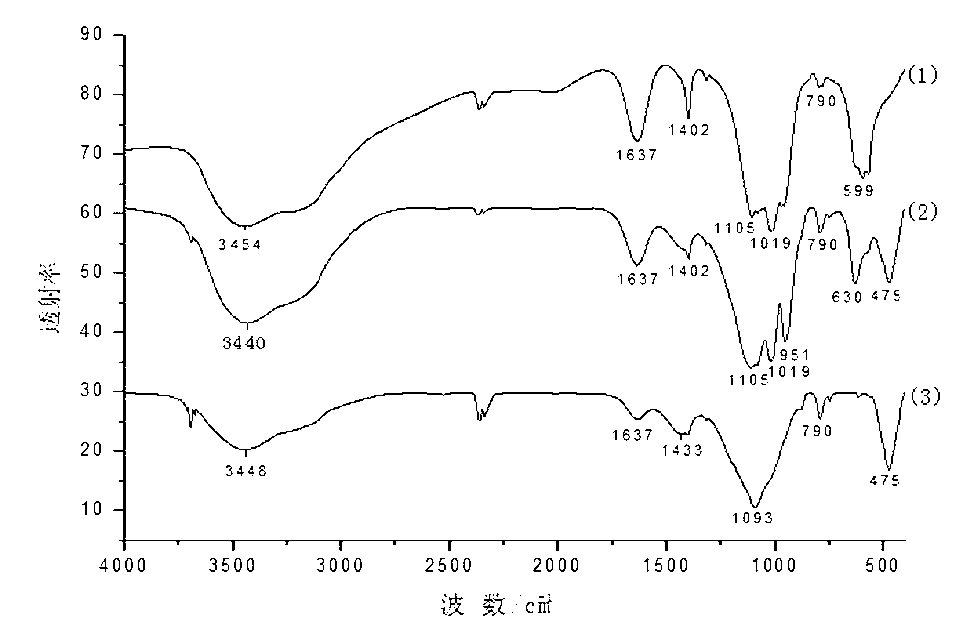
[0032] (3) Adjust the pH of the suspension C to 7.2 with an appropriate amount of ammonia water, filter the suspension C and bake the solid powder at 105°C for 2 hours to obtain an intumescent flame retardant synergist with a yield of 89%. The obtained product was subjected to XRD analysis and FTIR analysis.
[0033] attached ...
Embodiment 2
[0036] For the preparation process, see the attached figure 1
[0037] (1) Add 100mL of distilled water into a beaker, add 20g of dry magnesium oxide desulfurization waste residue, stir evenly at room temperature until the waste residue is completely wetted, and prepare a suspension A with a mass concentration of 20%;
[0038] (2) Add 0.08mol sodium dihydrogen phosphate to the above suspension A to obtain suspension B, stir and absorb at room temperature for 1 hour, then add 0.07mol ammonia water and 0.08mol zinc chloride to the above suspension B at the same time. The reaction was stirred at room temperature for 1 hour to obtain a suspension C;
[0039] (3) Adjust the pH of the suspension C to 7.1 with an appropriate amount of ammonia water, filter the suspension C and bake the solid powder at 105°C for 2 hours to obtain an intumescent flame retardant synergist with a yield of 90%.
Embodiment 3
[0041] For the preparation process, see the attached figure 1
[0042] (1) Add 100mL of distilled water into a beaker, add 30g of dry magnesia desulfurization waste residue, stir evenly at room temperature until the waste residue is completely wetted, and prepare suspension A with a mass concentration of 30%;
[0043] (2) Add 0.12 mol of sodium dihydrogen phosphate to the above suspension A to obtain suspension B, stir and absorb at room temperature for 1 hour, then add 0.1 mol of ammonia water and 0.12 mol of zinc chloride to the above suspension B at the same time. The reaction was stirred at room temperature for 1 hour to obtain a suspension C;
[0044] (3) Adjust the pH of the suspension C to 7.1 with an appropriate amount of ammonia water, filter the suspension C and bake the solid powder at 105°C for 2 hours to obtain an intumescent flame retardant synergist with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com