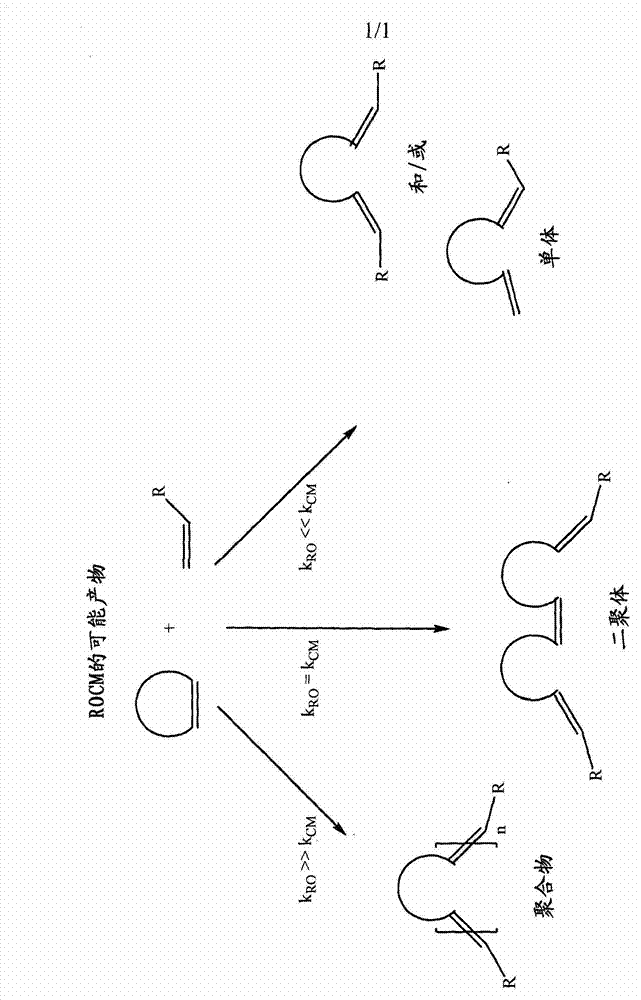
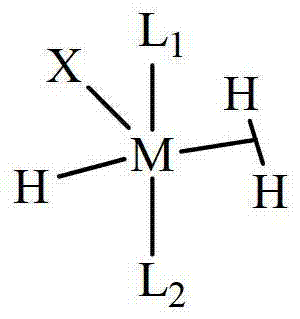
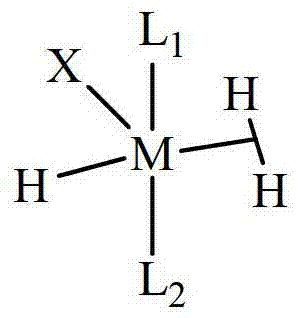
A novel class of olefin metathesis catalysts, methods of preparation, and processes for the use thereof
A technology for metathesis catalysts and olefins, which is applied in the directions of catalyst activation/preparation, metathesis reaction to hydrocarbons, catalysts, etc., and can solve the problems of high cost and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Example 1: ROMP of DCPD: 10.0 g (75.6 mmol) of dicyclopentadiene was added to a 20 ml scintillation vial. Add 5.0 mg (7.2 μmol) of RuClH (H 2 )(PCy 3 ) 2 . per equivalent of RuClH(H 2 )(PCy 3 ) 2 1 equivalent of phenylacetylene and hexachloroethane were fed via addition of 0.1 mls of each of 72 mM stock solutions of phenylacetylene and hexachloroethane. The dicyclopentadiene ring-opening polymerization was observed by the solution gradually becoming thick to a rubbery viscous material after six minutes. After one hour, a solid rubber material formed.
Embodiment 2
[0269] Example 2: ROMP of DCPD: 10.0 g (75.6 mmol) of dicyclopentadiene was added to a 20 ml scintillation vial. Add 5.0 mg (7.2 μmol) of RuClH (H 2 )(PCy 3 ) 2 . per equivalent of RuClH(H 2 )(PCy 3 ) 2 5 equivalents of phenylacetylene and hexachloroethane were fed via addition of 0.5 mls of each of 72 mM stock solutions of phenylacetylene and hexachloroethane. The ring opening polymerization of dicyclopentadiene was observed by the rapid thickening of the solution to a rubbery viscous material. After 4 minutes, the material was a rubbery solid. After 24 hours, the material was a hard rubber solid.
Embodiment 3
[0270] Example 3: ROMP of DCPD: 10.0 g (75.6 mmol) of dicyclopentadiene was added to a 20 ml scintillation vial. Add 5.0 mg (7.2 μmol) of RuClH (H 2 )(PCy 3 ) 2 . per equivalent of RuClH(H 2 )(PCy 3 ) 2 1 equivalent of phenylacetylene was added via the addition of 0.1 mls of a 72 mM solution of phenylacetylene. per equivalent of RuClH(H 2 )(PCy 3 ) 2 10 equivalents of hexachloroethane were fed via the addition of 1.0 mls of a 72 mM solution. Ring-opening polymerization was observed as the solution gradually thickened to a rubbery viscous material after six minutes. After 1 hour, a solid rubber material had formed.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap