Method for controlling prekallikrein activator in human serum albumin product
A technology of human albumin and kallikrein, which is applied in the field of preparation of human albumin products, can solve the problems of high prokallikrein activator, etc., and achieve the effect of reducing PKA content and ensuring reliable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
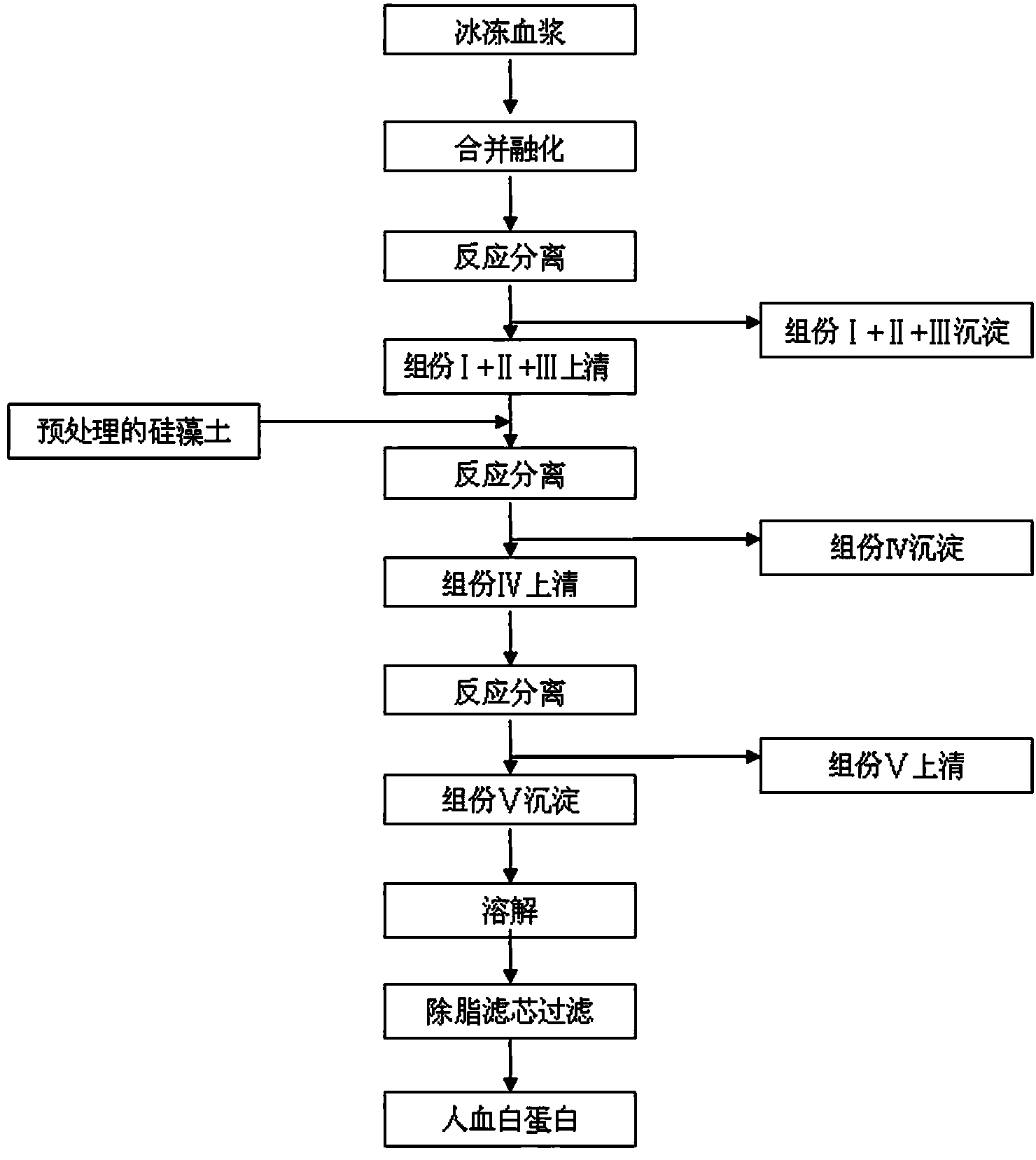
[0024] 1. Diatomite activation pretreatment
[0025] Dissolve 100g of sodium citrate in 50L of water for injection at 20-30°C, add 5kg of diatomaceous earth, stir for 5 minutes, suspend, and let stand for 5 minutes to remove suspended matter and supernatant;
[0026] Repeat the above operation with water for injection without sodium citrate and drain it repeatedly until the pH of the lotion is neutral;
[0027] Dissolve 25 mL of glacial acetic acid in 10L of 20-30°C water for injection, add the above-mentioned drained solid, stir for 5 minutes, suspend, stand for 3 minutes, drain to obtain a solid;
[0028] Wash the solid repeatedly with water for injection until the pH of the washing solution is neutral, drain it to obtain activated diatomaceous earth, and place it in a cold operating room for later use.
[0029] 2. Adsorption of coagulation factor Ⅻ
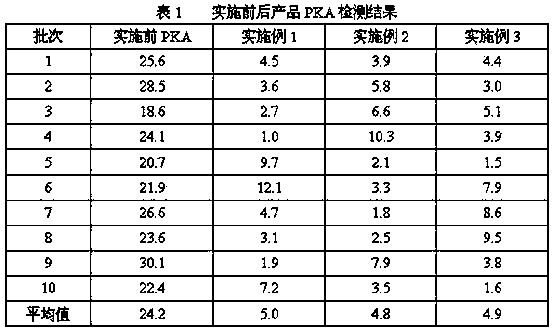
[0030] Take 100L of the fraction Ⅰ+Ⅱ+Ⅲ supernatant obtained in the process of separating and preparing human albumin by the low-temperat...
Embodiment 2
[0035] 1. Diatomite activation pretreatment
[0036] Dissolve 100g of sodium citrate in 50L of water for injection at 20-30°C, add 5kg of diatomaceous earth, stir for 8 minutes, suspend, and let stand for 4 minutes to remove suspended matter and supernatant;
[0037] Repeat the above operation with water for injection without sodium citrate and drain it repeatedly until the pH of the lotion is neutral;
[0038] Dissolve 25mL of glacial acetic acid in 10L of 20-30°C water for injection, add the above-mentioned drained solid, stir for 8 minutes, suspend, stand for 4 minutes, drain to obtain a solid;
[0039] Wash the solid repeatedly with water for injection until the pH of the washing solution is neutral, drain it to obtain activated diatomaceous earth, and place it in a cold operating room for later use.
[0040] 2. Adsorption of coagulation factor Ⅻ
[0041] Take 100L of component Ⅰ+Ⅱ+Ⅲ supernatant obtained in the process of separating and preparing human albumin by low-temperature etha...
Embodiment 3
[0046] 1. Diatomite activation pretreatment
[0047] Dissolve 100g of sodium citrate in 50L of water for injection at 20-30°C, add 5kg of diatomaceous earth, stir for 10 minutes, suspend, and let stand for 5 minutes to remove suspended matter and supernatant;
[0048] Repeat the above operation with water for injection without sodium citrate and drain it repeatedly until the pH of the lotion is neutral;
[0049] Dissolve 25 mL of glacial acetic acid in 10L of 20-30°C water for injection, add the above-mentioned drained solid, stir for 10 minutes, suspend, let stand for 5 minutes, drain to obtain a solid;
[0050] Wash the solid repeatedly with water for injection until the pH of the washing solution is neutral, drain it to obtain activated diatomaceous earth, and place it in a cold operating room for later use.
[0051] 2. Adsorption of coagulation factor Ⅻ
[0052] Take 100L of the fraction Ⅰ+Ⅱ+Ⅲ supernatant obtained in the process of separating and preparing human albumin by the low-te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com