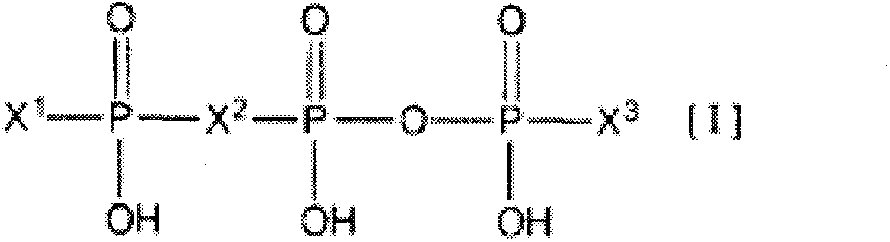
Agent for treatment of dry eye characterized by combining P2Y2 receptor agonist with hyaluronic acid or salt thereof, method for treating dry eye, and use of the P2Y2 receptor agonist and hyaluronic acid or salt thereof
一种受体激动剂、透明质酸的技术,应用在干眼症的治疗,制造干眼症治疗剂中的应用,眼科用剂,P2Y2受体激动剂和透明质酸或其盐领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
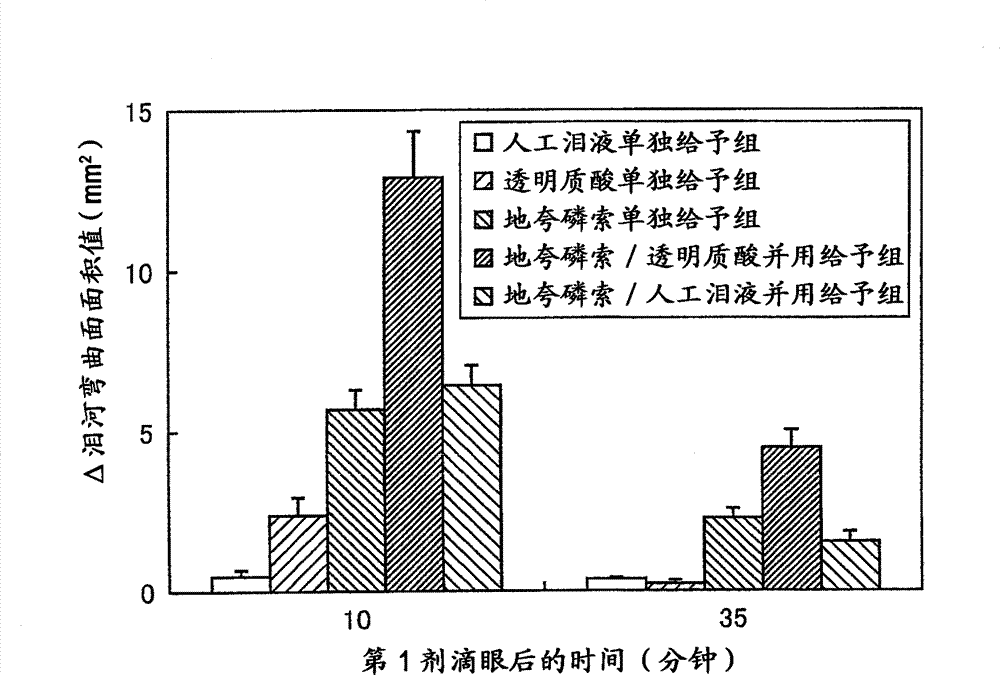
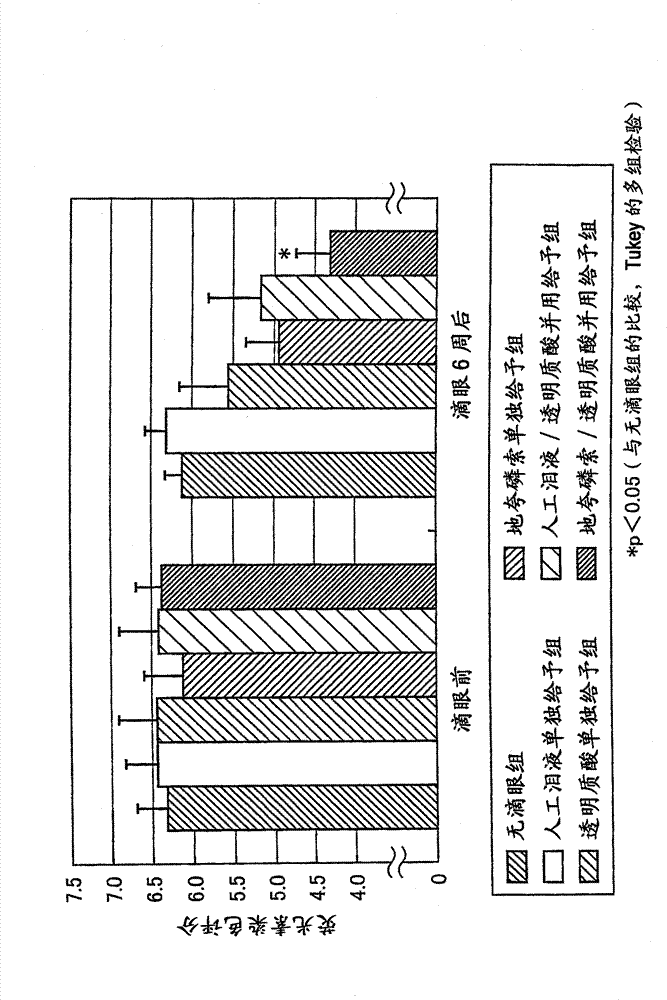
[0180] [Pharmacological Test 1]
[0181] Changes in tear fluid storage can be evaluated by measuring the area of the curved surface of the tear river stained with a fluorescein solution (Exp. Eye. Res., 78(3), 399-407(2004)). Therefore, according to the method of Murakami et al. (Ophthalmic Res., 34, 371-374 (2002)), normal male white rabbits were used to evaluate as P2Y 2 Time-dependent changes in the area of the lacrimal surface after continuous instillation of receptor agonists diquafosol sodium and sodium hyaluronate.
[0182] (drug preparation method)
[0183] Dissolve 30 mg of diquafosol sodium in phosphate buffer, add potassium chloride, sodium chloride, benzalkonium chloride, and a pH regulator to prepare 1 mL of an isotonic and neutral aqueous solution, which is used as 3% diquafosol Phosphorus sodium eye drops were used in this experiment. It should be noted that "soft santear" manufactured by Santen Pharmaceutical Co., Ltd. was used as artificial tears, and "...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com