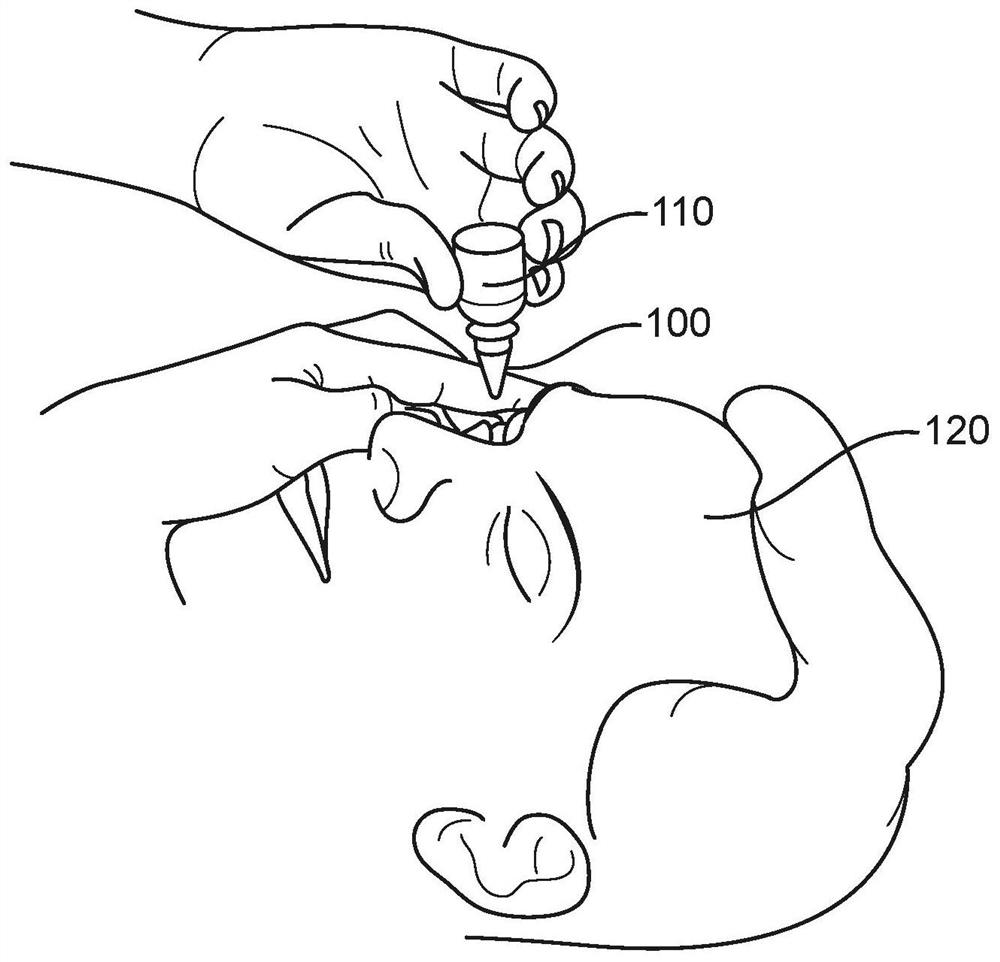
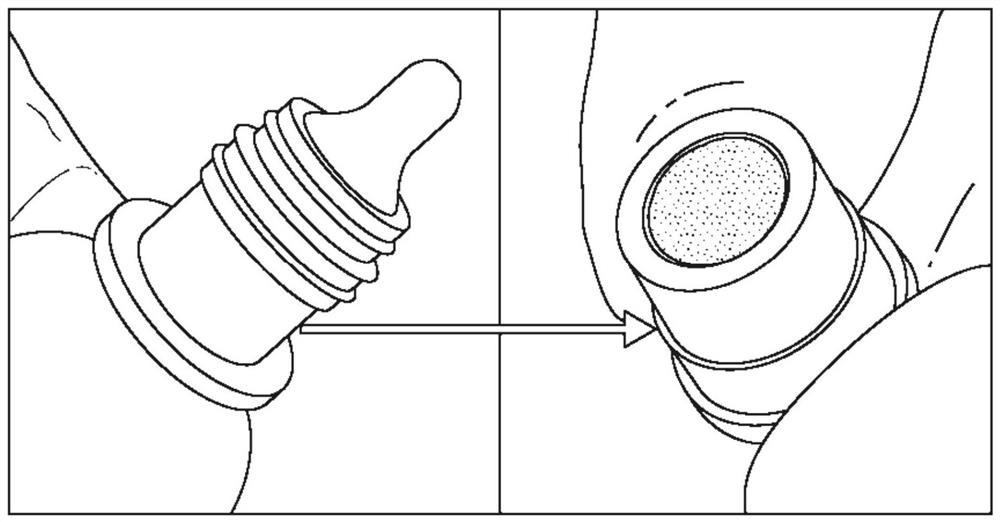
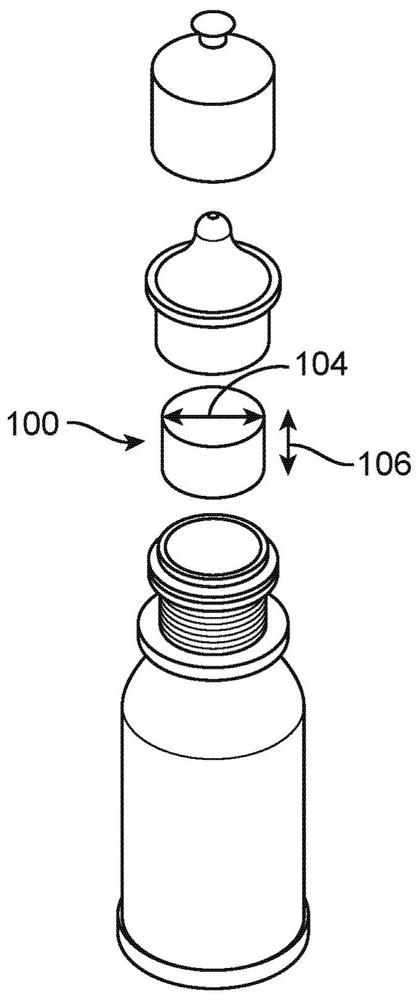
Systems and methods for preservative removal from ophthalmic formulations comprising complexing agents
A complexing agent and preservative technology, applied in the field of removing preservatives and applying ophthalmic reagents, can solve problems such as dosage changes and reducing the shelf life of eye drop preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] The concentration of latanoprost in the droplets of solution A that passed through the porous polymer hydrogel B was at least 80% of the original concentration of latanoprost in solution A. The droplets more preferably have 90% of the original concentration of latanoprost in solution A. And most preferably >95% of the original concentration of latanoprost in solution A.
[0160] Furthermore, the concentration of total BAK in droplets of solution A passing through porous polymer hydrogel B was less than 50% of the original solubility of BAK in solution A. The droplets more preferably have less than 20% of the original concentration of BAK in solution A, and even more preferably have less than 5% of the original concentration of BAK in solution A. Most preferably have <1% of the original concentration of BAK in solution A or below the detection limit of one skilled in the art of the original concentration of BAK in solution A.
[0161] Furthermore, the concentration of ...
Embodiment
[0166] It should be understood that the examples and embodiments described herein are for illustrative purposes only and are not intended to limit the scope of the claimed invention. It should also be understood that from the examples and embodiments described herein, various modifications or changes will occur to those skilled in the art, which are included within the spirit and scope of the present application and the scope of the appended claims. All publications, patents, and patent applications cited herein are hereby incorporated by reference in their entirety for all purposes.
[0167] It should be understood that various ophthalmic agents can be used in any aspect of the provided disclosure. It should be understood that various cyclodextrins can be used in any aspect of the provided disclosure to complex ophthalmic agents in aqueous solution. It should be understood that various preservatives can be used in any aspect of the provided disclosure to make the original so...
Embodiment 2
[0182] Comparative solution B (without CD) was prepared in the following manner.
[0183] 0.1 gm (2.313 × 10 -4 mol) latanoprost was mixed with 2000 mL of distilled water and stirred at high speed under nitrogen atmosphere for several hours to ensure complete dissolution. 0.4 gm of benzalkonium chloride (BAK) was added to the solution and mixing was continued at 25°C to ensure a homogeneous clear solution. The concentration of latanoprost in solution B was 0.005% and the BAK was 0.02% by weight.
[0184] US Patent No. 10,123,904, which is incorporated herein by reference in its entirety, previously described a procedure demonstrating selective absorption of BAK preservatives from Solution B (both prepared as described herein) through porous polymeric hydrogel B. Another procedure (analytical method) is to use quantitative HPLC using the partition coefficient method or a simple equilibration test to compare the area under the curve (AUC) of the starting solution of drug and B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com