Medicinal preparation
A preparation and medicine technology, applied in the field of pharmaceutical preparations, can solve the problem of no record of stability, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0223] Below, the present invention is further described by enumerating examples, but the present invention is not limited by them.
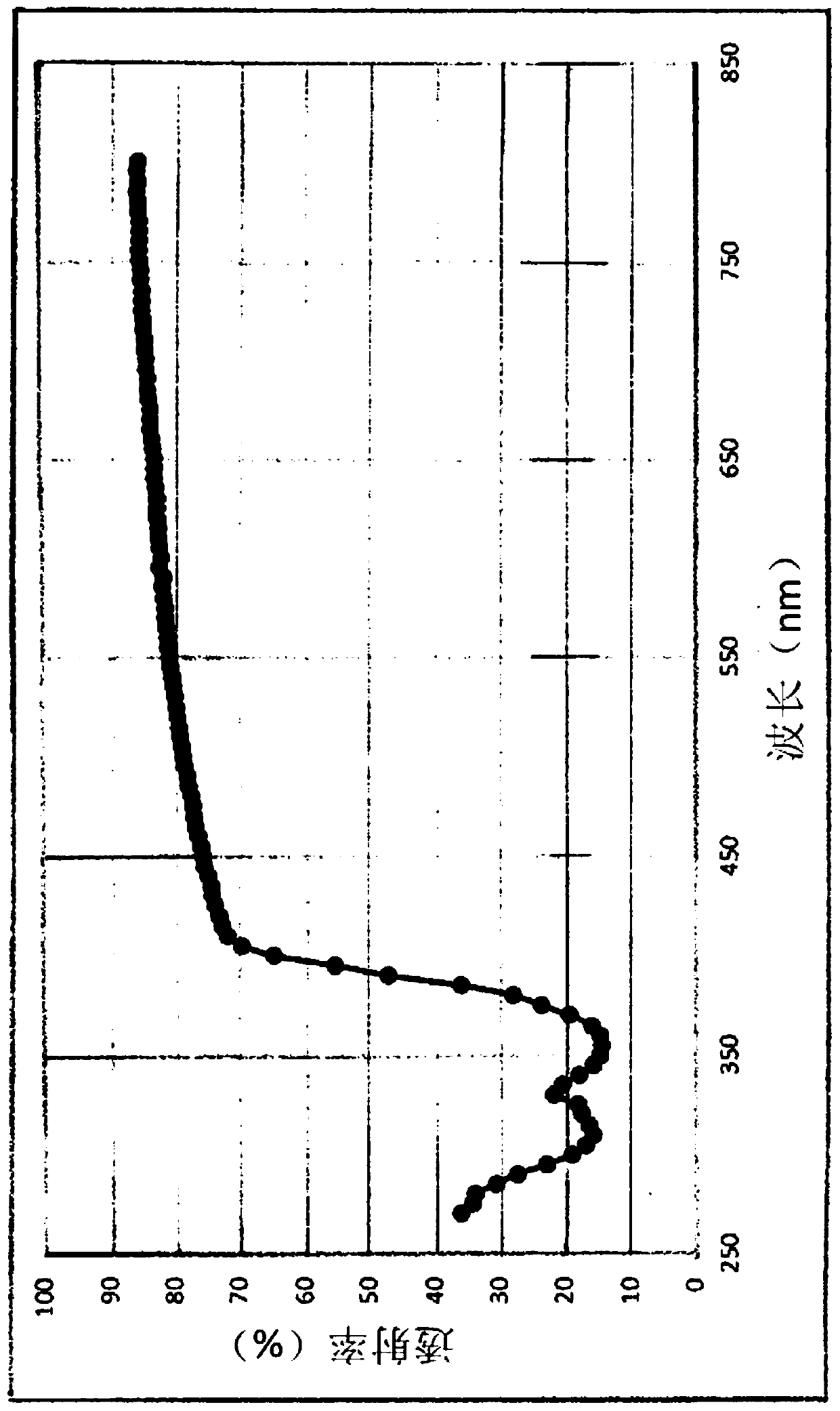
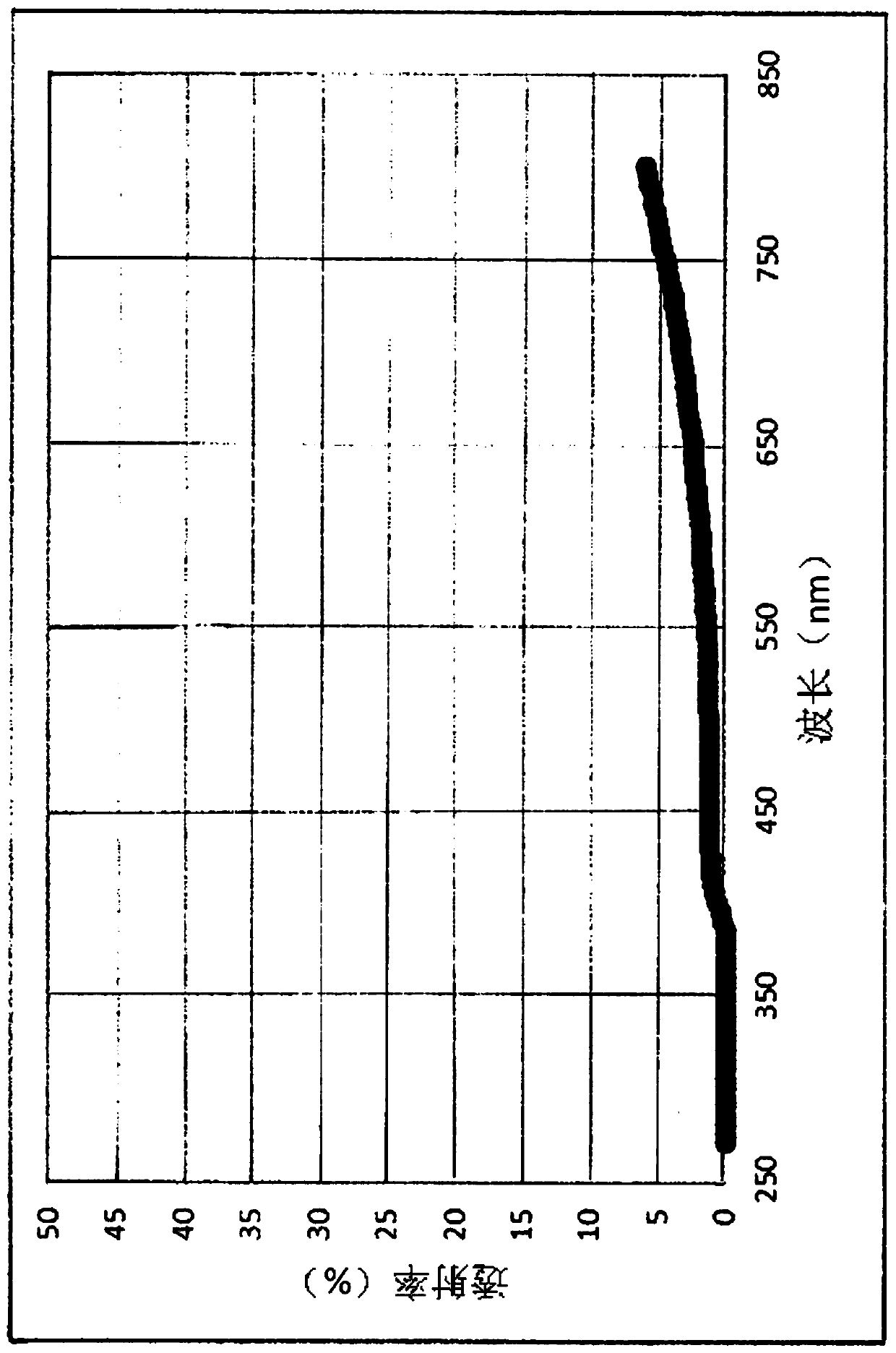
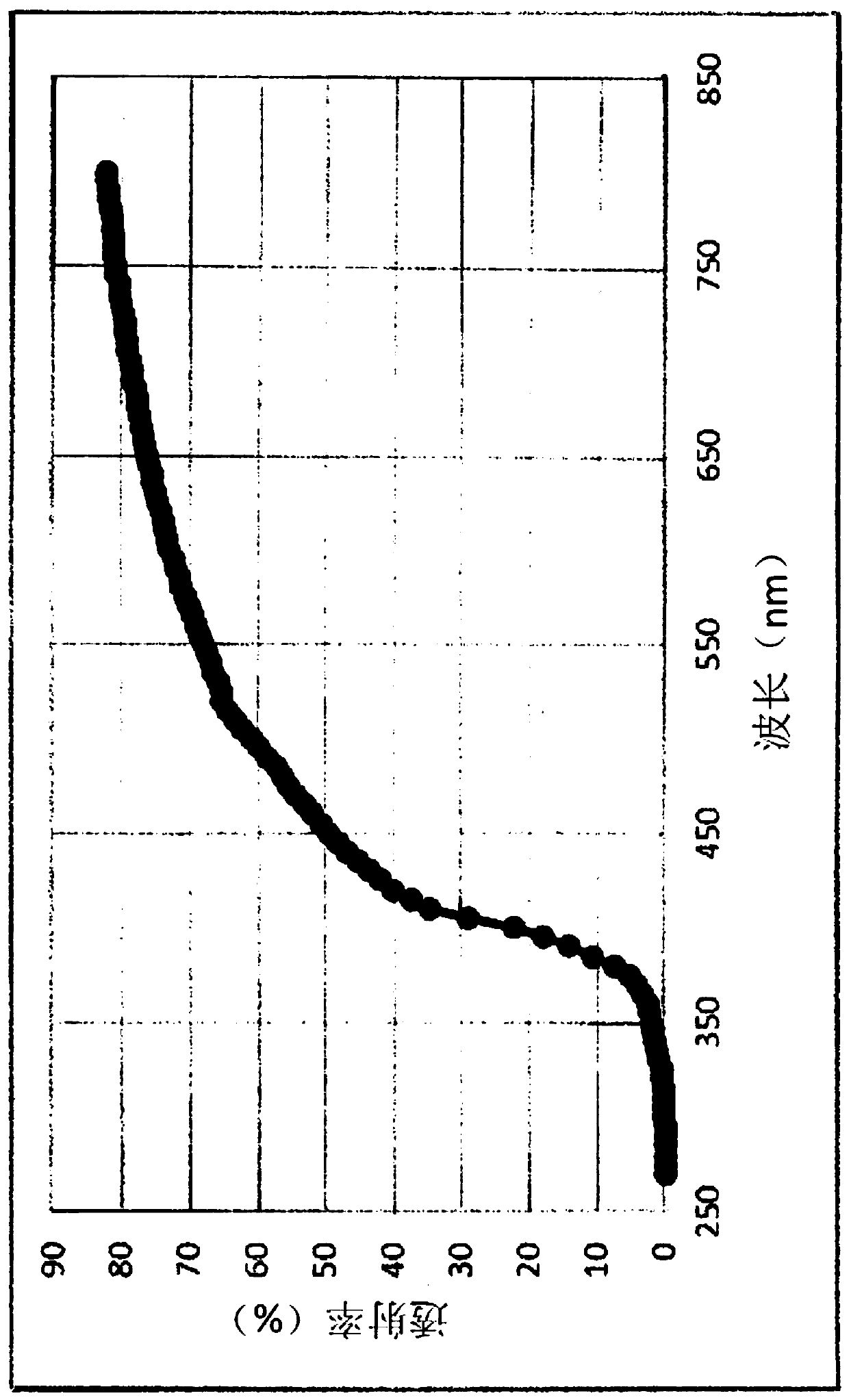
[0224] In addition, in the following test examples, in the measurement of netadil using HPLC, an ODS column was used as a column, 0.01 mol / L phosphate buffer and acetonitrile were used as a mobile phase, and an ultraviolet absorptiometry (wavelength : 254nm).
manufacture example 1~36
[0300] Aqueous compositions containing the ingredients and amounts (amount (g) per 100 mL of the aqueous composition) described in Tables 9 to 17 were prepared in accordance with a conventional method, and contained in Test Example 3 using a mixture containing benzotriazole The container (primary package) for eye drops made of polypropylene, which is an ultraviolet absorber, can produce the pharmaceutical preparations of Production Examples 1 to 36.
[0301] [Table 9]
[0302]
[0303] [Table 10]
[0304]
[0305] [Table 11]
[0306]
[0307] [Table 12]
[0308]
[0309] [Table 13]
[0310]
[0311] [Table 14]
[0312]
[0313] [Table 15]
[0314]
[0315] [Table 16]
[0316]
[0317] [Table 17]
[0318]
manufacture example 37~72
[0320] In Production Examples 1 to 36, except that the container was made of polyethylene instead of polypropylene, the pharmaceutical preparations of Production Examples 37 to 72 could be produced using the same container as that used in Test Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com