Ras and HDAC dual inhibitor as well as preparation method and application thereof
A dual inhibitor, CH2 technology, used in the preparation of thioethers, anti-inflammatory agents, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
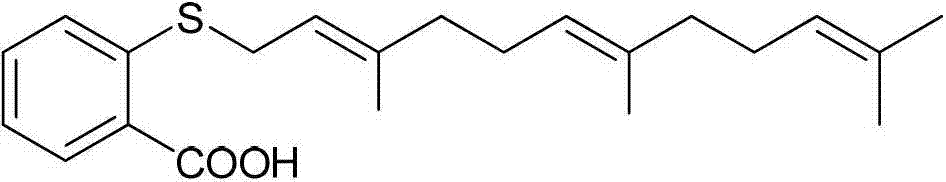
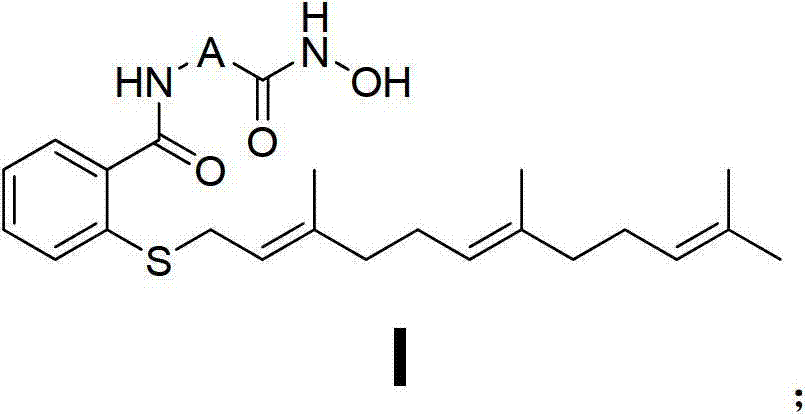
[0101] Embodiment 1N-(2-(hydroxylamino)-2-oxaethyl)-farnesyl thiosalicylic acid amide (Ⅰ 1 ) Preparation of farnesylthiosalicylic acid chloride (1)
[0102] Dissolve 0.36 g (1.00 mmol) FTA in 10 mL anhydrous CH 2 Cl 2 0.40 mL (5.51 mmol) of thionyl chloride was added thereto, stirred at 55°C for 1 hour, and concentrated to obtain farnesylthiosalicylic acid chloride (1) as a yellow oil.
[0103] Preparation of N-(2-(methoxy)-2-oxaethyl)-farnesylthiosalicylic acid amide (2a)
[0104] Dissolve 0.09 g (1.00 mmol) of glycine methyl ester and 0.2 mL (1.50 mmol) of triethylamine in 5 mL of anhydrous CH 2 Cl 2 , add dropwise 10 mL of anhydrous CH prepared in 1 under ice bath 2 Cl 2 solution, then stirred at room temperature for 1.5h, the reaction solution was washed with 10mL water and saturated NaCl solution, CH 2 Cl 2 Dry with anhydrous sodium sulfate, filter, and spin dry to obtain 0.37g of yellow oil, with a yield of 86%.
[0105] N-(2-(hydroxyamino)-2-oxaethyl)-farnesylt...
Embodiment 2
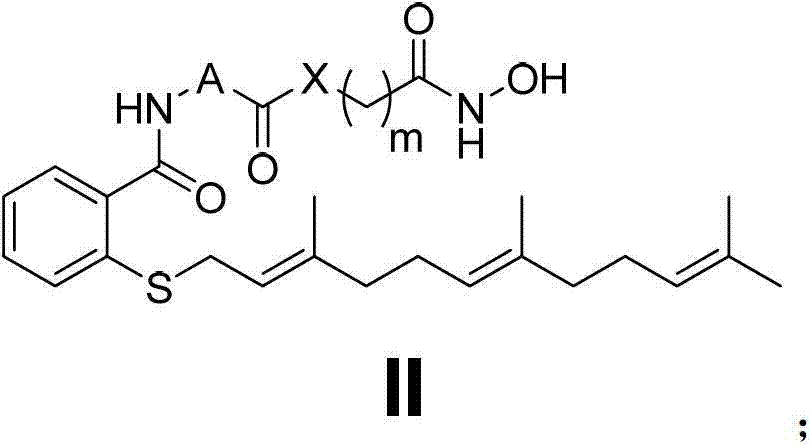
[0108] Embodiment 2N-(3-(hydroxylamino)-3-oxapropyl group)-farnesyl thiosalicylic acid amide (Ⅰ 2 ) preparation
[0109] Preparation of N-(3-(methoxy)-3-oxapropyl)-farnesylthiosalicylic acid amide (2b)
[0110] With reference to the preparation method of N-(2-(methoxy)-2-oxaethyl)-farnesylthiosalicylic acid amide (2a) in Example 1, the method is replaced by methyl 3-alanine Glycine methyl ester in (1) was reacted with (1) to obtain yellow oily substance (2b) with a yield of 85%.
[0111] N-(3-(hydroxyamino)-3-oxapropyl)-farnesylthiosalicylic acid amide (Ⅰ 2 ) preparation
[0112] Referring to I in Example 1 1 The preparation method, by 2a in the alternative method of 2b, reacts with hydroxylamine hydrochloride to obtain oil (Ⅰ 2 ), with a yield of 73%.
[0113] 1 H NMR (CDCl 3 ,300MHz):δ8.24(m,1H,NH),8.09(m,1H,NH),7.65(m,2H,ArH),7.37(m,2H,ArH),5.41(m,1H,SCH 2 C H ),5.18(m,2H,2×CH 2 C H =CCH 3 ), 3.76(d, 2H, J=7.2Hz, SC H 2 ),3.18(m,4H,2×CH 2 ),2.05-1.83(m,8H,2×...
Embodiment 3
[0114] Embodiment 3N-(4-(hydroxylamino)-4-oxabutyl)-farnesyl thiosalicylic acid amide (Ⅰ 3 ) preparation
[0115] Preparation of N-(4-(methoxy)-4-oxabutyl)-farnesylthiosalicylic acid amide (2c)
[0116] With reference to the preparation method of N-(2-(methoxy)-2-oxaethyl)-farnesylthiosalicylic acid amide (2a) in Example 1, the method is replaced by methyl 4-aminobutyrate Glycine methyl ester in (1) was reacted with (1) to obtain yellow oil (2c), the yield was 83%.
[0117] N-(4-(hydroxyamino)-4-oxabutyl)-farnesylthiosalicylic acid amide (Ⅰ 3 ) preparation
[0118] Referring to I in Example 1 1 The preparation method, by 2a in the alternative method of 2c, reacts with hydroxylamine hydrochloride to obtain oil (Ⅰ 3 ), the yield is 70%.
[0119] 1 H NMR (CDCl 3 ,300MHz):δ7.98(d,1H,J=7.8Hz,Ar-H),7.60-7.57(m,2H,Ar-H),7.39(m,1H,Ar-H),5.46(m, 1H,SCH 2 C H ),5.20(m,2H,2×CH 2 C H =CCH 3 ),3.78(d,2H,J=7.2Hz,SC H 2 ),3.52(m,2H,NHC H 2 ),2.34(m,2H,C H 2 CONH),2.21(m,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com