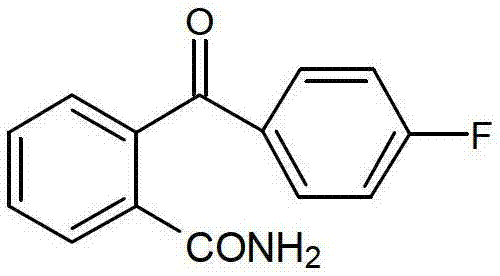
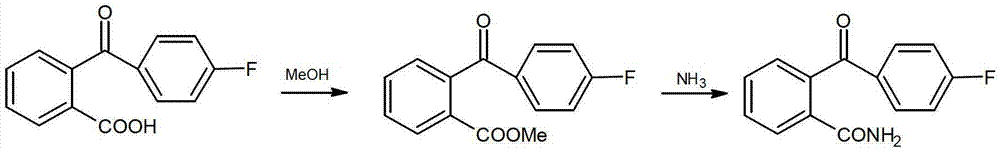
Method for preparing 2-(4-fluorobenzene formyl) benzamide
A technology of fluorobenzoyl and benzamide, which is applied in the field of preparation of 2-benzamide, can solve the problems of serious environmental pollution, inconvenient use, and high equipment requirements, and achieves low environmental pollution, few by-products, and equipment The effect of less corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of preparation method of 2-(4-fluorobenzoyl) benzamide, its step is as follows:
[0025] 1) Esterification Add 48.8g of 2-(4-fluorobenzoyl)benzoic acid into 420ml of dichloromethane, stir and dissolve, then add 16g of methanol and 0.016g of concentrated sulfuric acid, and reflux for 4.5h;
[0026] 2) After esterification treatment, add 20% sodium hydroxide aqueous solution to the above-mentioned reaction system, adjust the pH value to 6, distill off the remaining methanol in the reaction, add appropriate amount of water and dichloromethane, and let stand to separate layer, the aqueous phase was extracted 4 times with dichloromethane, the organic phases were combined, and the organic phase was washed with saturated brine until the pH of the washing liquid was 7, then dried with anhydrous magnesium sulfate for one hour, filtered, and the filtrate was evaporated under normal pressure Solvent, after the solvent is evaporated to dryness, 41.16g2-(4-fluorobenzoyl) meth...
Embodiment 2
[0030] A kind of preparation method of 2-(4-fluorobenzoyl) benzamide, its step is as follows:
[0031] 1) Esterification Add 48.8g of 2-(4-fluorobenzoyl)benzoic acid into 420ml of dichloromethane, stir and dissolve, then add 22.4g of methanol and 0.04g of concentrated sulfuric acid, and reflux for 4 hours;
[0032] 2) After esterification treatment, add 20% sodium hydroxide aqueous solution to the above-mentioned reaction system, adjust the pH value to 7, distill off the remaining methanol in the reaction, add appropriate amount of water and dichloromethane, and let stand to separate layer, the aqueous phase was extracted 4 times with dichloromethane, the organic phases were combined, and the organic phase was washed with saturated brine until the pH of the washing liquid was 7, then dried with anhydrous magnesium sulfate for one hour, filtered, and the filtrate was evaporated under normal pressure Solvent, after the solvent is evaporated to dryness, 36.32g2-(4-fluorobenzoyl) ...
Embodiment 3
[0036] A kind of preparation method of 2-(4-fluorobenzoyl) benzamide, its step is as follows:
[0037] 1) Esterification Add 48.8g of 2-(4-fluorobenzoyl)benzoic acid into 420ml of dichloromethane, stir to dissolve, add 19.2g of methanol and 0.06g of concentrated sulfuric acid, and reflux for 4.2h;
[0038] 2) After esterification treatment, add 20% sodium hydroxide aqueous solution to the above-mentioned reaction system, adjust the pH value to 6.5, distill off the remaining methanol in the reaction, add appropriate amount of water and dichloromethane, and let stand to separate layer, the aqueous phase was extracted 4 times with dichloromethane, the organic phases were combined, and the organic phase was washed with saturated brine until the pH of the washing liquid was 7, then dried with anhydrous magnesium sulfate for one hour, filtered, and the filtrate was evaporated under normal pressure Solvent, after the solvent is evaporated to dryness, 38.74g2-(4-fluorobenzoyl) methyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com