Heteronuclear ruthenium-gold bicyclic metal compound and its preparation method and use
A compound and cyclic metal technology, applied in the field of heteronuclear ruthenium gold bicyclic metal compounds, can solve the problems of few reports of heteronuclear cyclic metal compounds, and achieve the effects of improving atom economy and synthesis efficiency, easy preparation and high catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
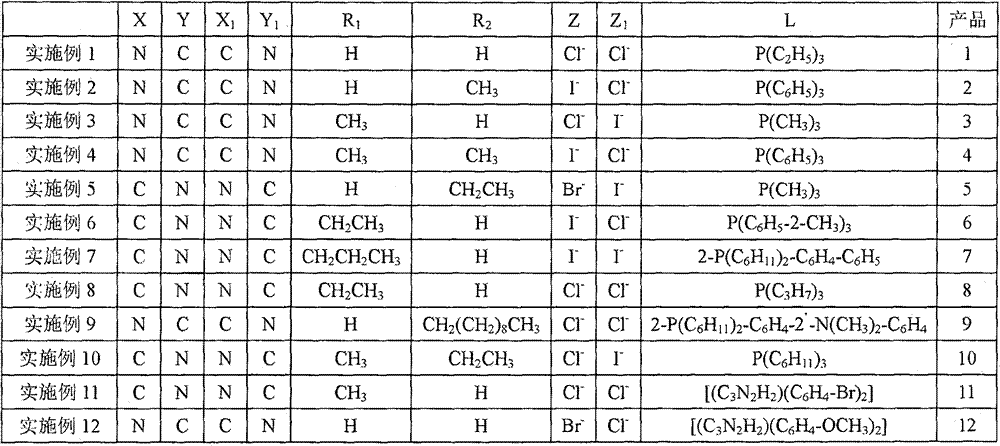
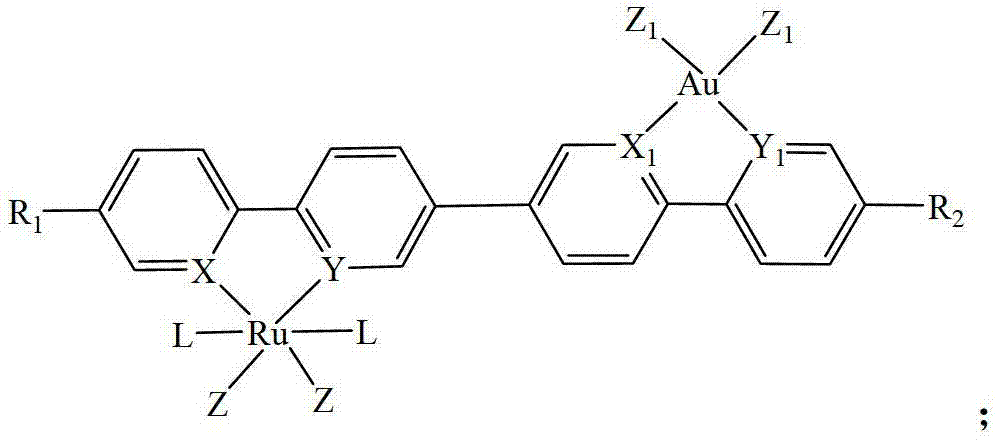
[0046] Example 1
[0047] The preparation method of the heteronuclear ruthenium-gold bicyclic metal compound in this embodiment is: adding triethylphosphine mononuclear ring ruthenium compound (1.05mmol) containing chlorine atom and containing boron into a 50ml three-necked flask equipped with a stirring reflux device The mononuclear ring gold compound of the acid ester group (1mmol), palladium chloride (0.10mmol), potassium phosphate (3.0mmol) and 20ml of anhydrous toluene were stirred and reacted for 20 hours under a nitrogen atmosphere at a temperature of 110°C and filtered. After evaporating the solvent, use dichloromethane (CH 2 Cl 2 1) Recrystallized with a mixed solvent of petroleum ether to obtain a yellow product 1 heteronuclear triethylphosphine ruthenium gold bicyclic metal compound with a yield of 86.8%. NMR analysis of the obtained product, the data are as follows: 1 H NMR: δ=8.86 (d, 1H, Ph-H), 8.61 (d, 1H, Ph-H), 8.15 (s, 1H, Ph-H), 7.66-7.32 (m, 11H, Ph-H) ,1.32(...
Example Embodiment
[0048] Example 2
[0049] The preparation method of the heteronuclear ruthenium-gold bicyclic metal compound in this embodiment is: adding triphenylphosphine mononuclear ring ruthenium compound containing iodine atom (1.2mmol) and containing boron into a 50ml three-necked flask equipped with a stirring reflux device The mononuclear ring gold compound of the acid ester group (1mmol), palladium acetate (0.05mmol), sodium phosphate (2.0mmol) and 20ml of anhydrous dioxane were stirred and reacted for 12 hours under a nitrogen atmosphere at a temperature of 110℃ Filter, evaporate the solvent and use dichloromethane (CH 2 Cl 2 ) Recrystallized in a mixed solvent with petroleum ether to obtain a yellow product 2 heteronuclear triphenylphosphine ruthenium gold bicyclic metal compound with a yield of 88.1%. NMR analysis of the obtained product, the data are as follows: 1 H NMR: δ=8.63 (d, 1H, Ph-H), 8.58 (d, 1H, Ph-H), 8.12 (d, 2H, Ph-H), 7.69-7.40 (m, 20H, Ph-H) ,7.11-7.39(m,19H,Ph-H),2...
Example Embodiment
[0050] Example 5
[0051] The preparation method of the heteronuclear ruthenium-gold bicyclic metal compound in this embodiment is: adding a trimethylphosphine mononuclear ring ruthenium compound containing bromine atoms (1.1mmol) and containing boron into a 50ml three-necked flask equipped with a stirring reflux device The mononuclear ring gold compound of the acid ester group (1mmol), palladium acetate (0.08mmol), potassium carbonate (3.0mmol) and 20ml of anhydrous tetrahydrofuran were stirred and reacted for 10 hours under a nitrogen atmosphere at a temperature of 80℃, then filtered and evaporated Use CH after solvent 2 Cl 2 It is recrystallized with a mixed solvent of petroleum ether to obtain a yellow product 5 heteronuclear trimethylphosphine ruthenium gold bicyclic metal compound with a yield of 92.6%. NMR analysis of the obtained product, the data are as follows: 1 H NMR: δ=8.56 (d, 1H, Ph-H), 8.53 (d, 1H, Ph-H), 8.11 (d, 2H, Ph-H), 7.63-7.45 (m, 8H, Ph-H) ,7.13(m,1H,Ph-...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap