Method for detecting esterification rate of heparin benzyl ester in production process of enoxaparin sodium
A technology of heparin benzyl ester and enoxaparin sodium, which is applied in the detection field of esterification rate, can solve the problems of difficult control of esterification rate, restriction of enoxaparin sodium production yield and product cost, etc., and facilitates industrialization and application , reduce the risk of non-conformity and the production cost of the enterprise, and overcome the effect that is difficult to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Instruments and utensils:
[0034] Liquid chromatography (VWD detector), electronic balance (1 / 100,000), volumetric flask (10mL, 25mL, 100mL), micropipette (1000μl)
[0035] 2. Reagents and reagents:
[0036] Methanol (chromatographically pure), acetonitrile (chromatographically pure), ultrapure water, benzyl alcohol standard, glacial acetic acid (analytical pure), sodium hydroxide (analytical pure)
[0037] 3. Solution preparation:
[0038] 3.1 Control solution: Accurately weigh 0.5g of benzyl alcohol standard product in a 1000ml volumetric flask, dissolve it with ultrapure water and dilute to the mark to obtain the product.
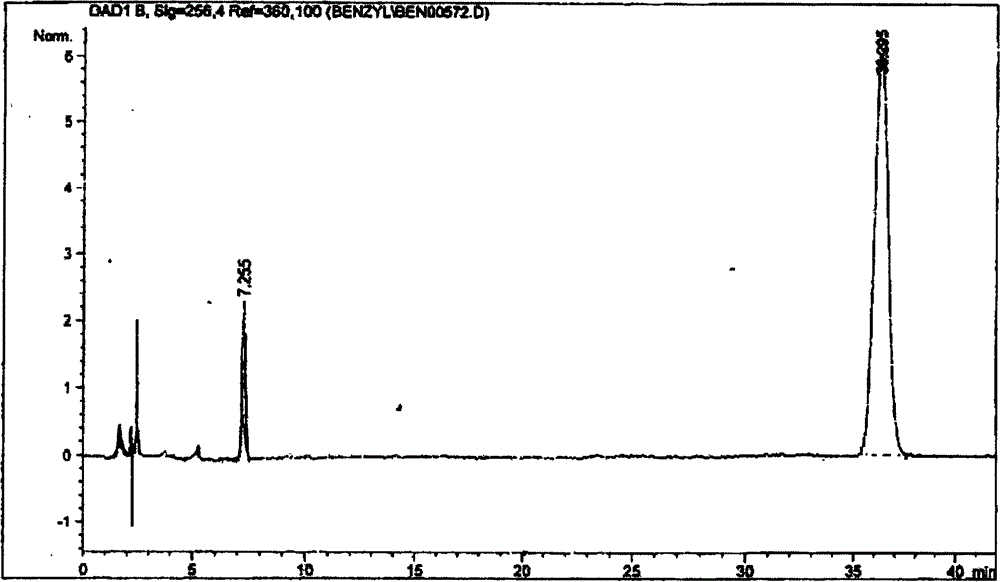
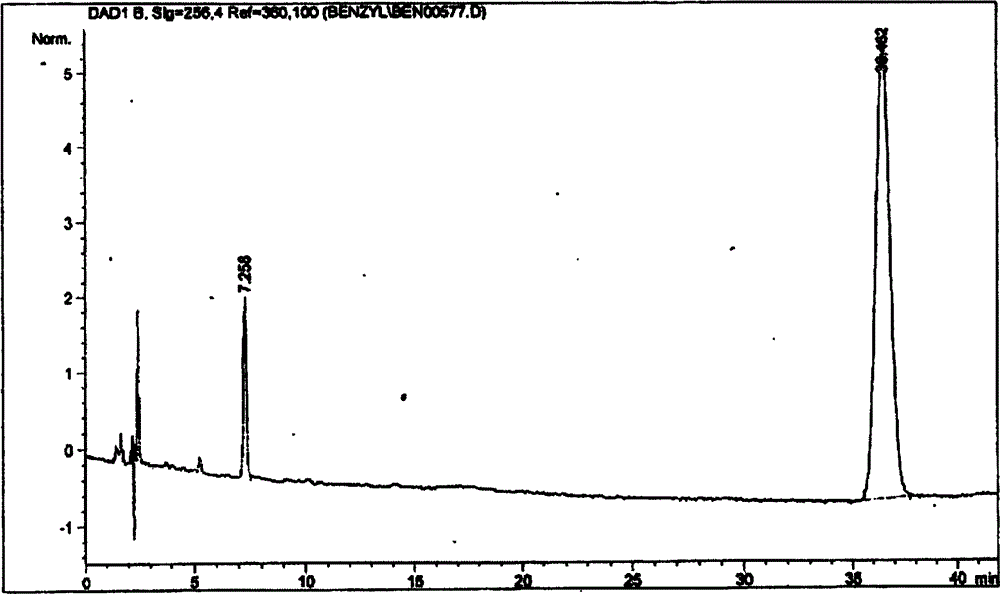
[0039] 3.2 Preparation of detection solution: Accurately weigh 0.5g of heparin benzyl ester sample (ZKE120709) into a 100ml volumetric flask, add 10ml of 5mol / L sodium hydroxide solution to dissolve, control the temperature in a water bath at 60-70°C, and react for 120 minutes. Add 10ml of glacial acetic acid to terminate the reaction, dilu...
Embodiment 2
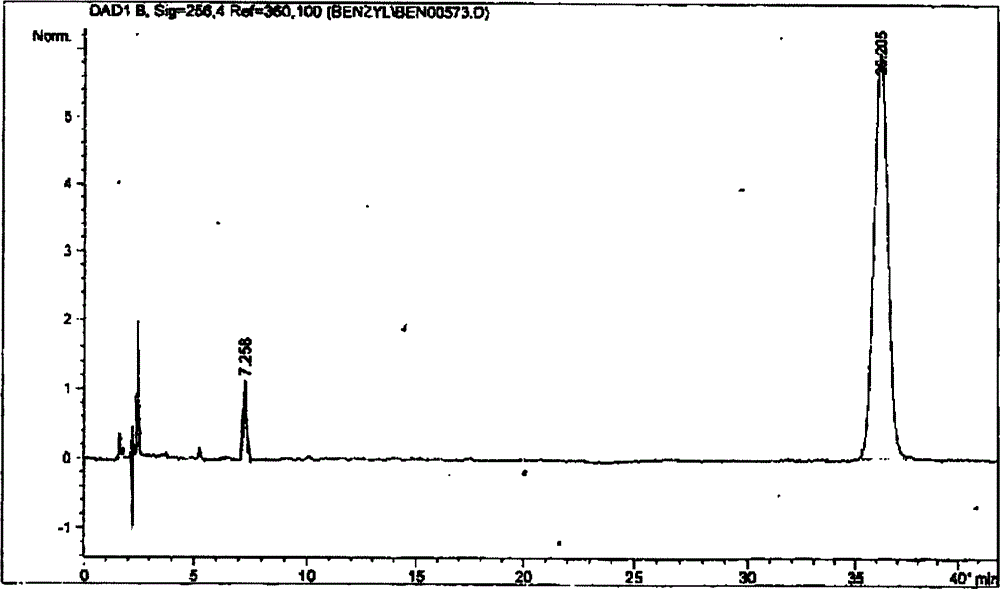
[0059] The heparin benzyl ester sample ZKE120715 was detected by the method described in Example 1.
[0060] E% = 1.8%.
[0061] It can be seen from the spectrogram:
[0062] A S =10.6
[0063] C S =4.8mg / ml
[0064] A R =22.2
[0065] C R =0.5mg / ml
[0066] Esterification rate (m / m) X = As × C R A R × C S × ( 1 - E % ) × 100 % = 10.6 × 0.5 22.2 × 4.8 × ( 1 - ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com