Method for detecting related substances of teriflunomide tablets
A technology of teriflunomide tablet and detection method, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problem of no teriflunomide tablet, etc., and achieve the effects of overcoming difficulty in control, simple detection method and high accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
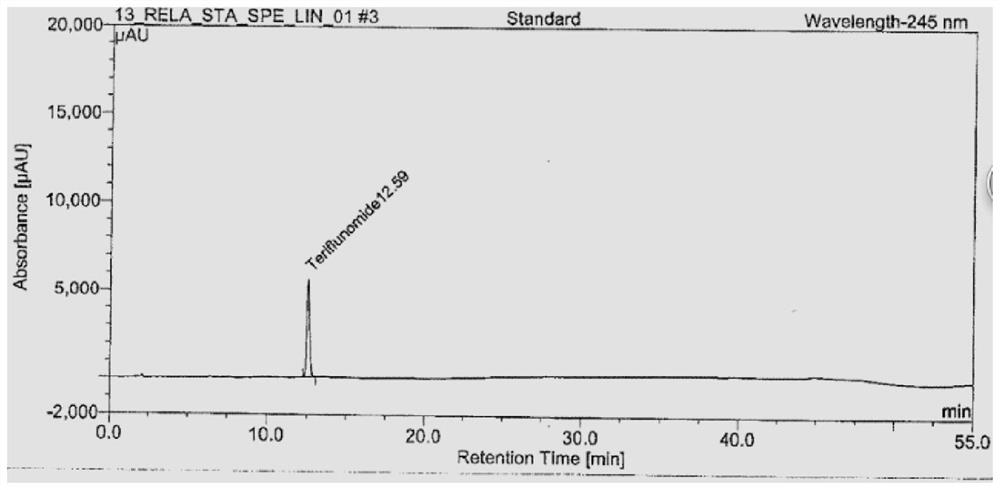
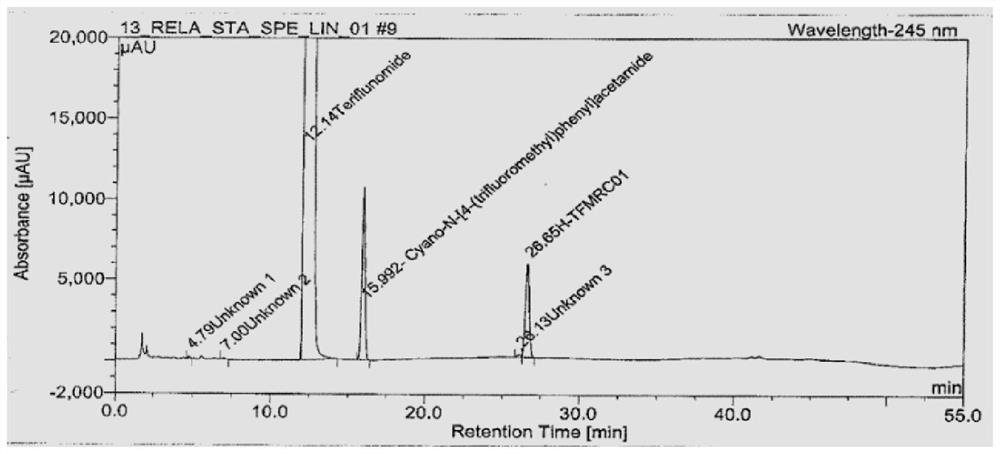
[0023] 1. Instruments and utensils: high performance liquid chromatography (UV detector), electronic balance (one millionth, one hundred thousandth), volumetric flask (50mL, 100mL, 500mL), pipette (2mL), centrifuge.
[0024] 2. Reagents and reagents: Potassium dihydrogen phosphate (analytical grade), acetonitrile (chromatographic grade), ultrapure water, teriflunomide reference substance (USP), phosphoric acid.
[0025] 3. Preparation of test solution:
[0026] 3.1 Diluent: Potassium dihydrogen phosphate solution, adjust the pH to 3.2±0.5 with phosphoric acid.
[0027] 3.2 Reference substance solution: Accurately weigh 28mg of teriflunomide reference substance into a 500mL volumetric flask, add diluent to dissolve to volume; pipette 2mL into a 100mL volumetric flask, add diluent to dissolve to volume, and shake well.
[0028] 3.3 Sample solution: Accurately weigh 2 tablets of teriflunomide into a 50mL volumetric flask, add about 30mL of diluent to ultrasonically dissolve, pl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com