Off-site regulation method used for medicine circulation
An off-site, pharmaceutical technology, applied in data processing applications, commerce, instruments, etc., can solve problems such as inability to provide early warning, difficulty in obtaining evidence, inability to recall, etc., and achieve the effects of improving scientific management, ensuring drug safety, and reliable technical support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
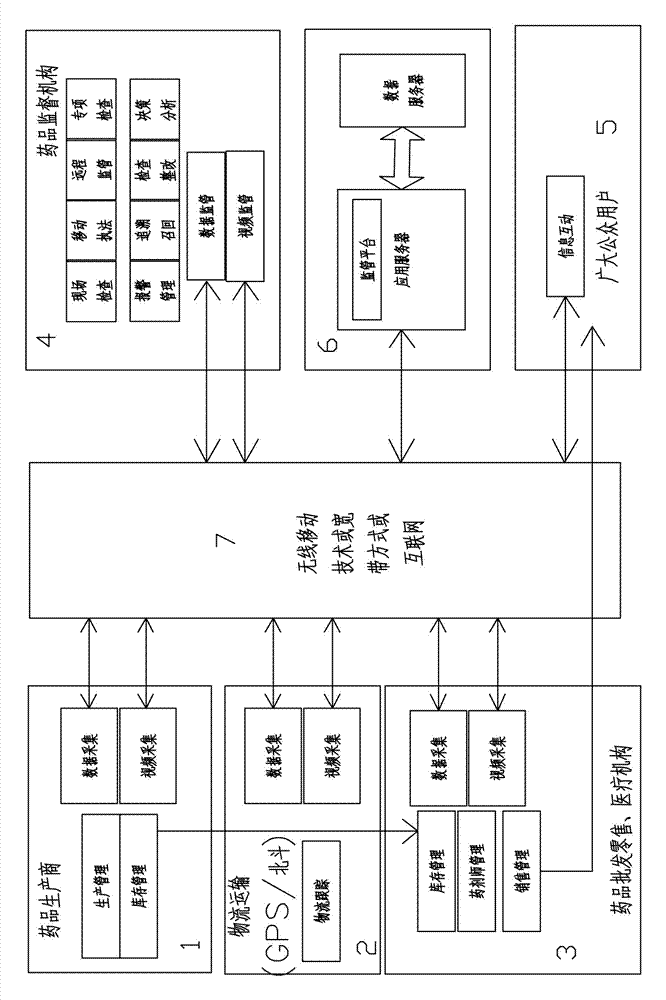
[0018] Such as figure 1 As shown, a method for off-site supervision of drug circulation is characterized in that it includes drug wholesale and retail or medical institutions 3, drug supervision agencies 4, data servers 6, wireless mobile technology or broadband methods or the Internet 7;
[0019] The retail or medical institution 3 includes inventory management, pharmacist management, sales management, data collection, video collection;
[0020] The drug supervision agency 4 includes on-site inspection, mobile law enforcement, remote supervision, special inspection, alarm management, food traceability, inspection and rectification, decision analysis, data supervision, and video supervision;
[0021] Described data server 6 comprises data server, application server; Described application server is provided with supervisory platform;
[0022] The drug wholesale and retail or medical institution 3 collects data and videos of the information of drug inspection, inspection, stora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com