Recombination porcine interferon gamma-Fc fusion protein as well as coding gene and expression method thereof
A fusion protein, porcine interferon technology, applied in chemical instruments and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of reducing the specific activity rate of recombinant proteins, insolubility, and unqualified product quality, etc. Achieve long-term effect and avoid repeated medication, prolong half-life, and control the effect of preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Recombinant porcine interferon gamma-Fc fusion protein gene optimization design
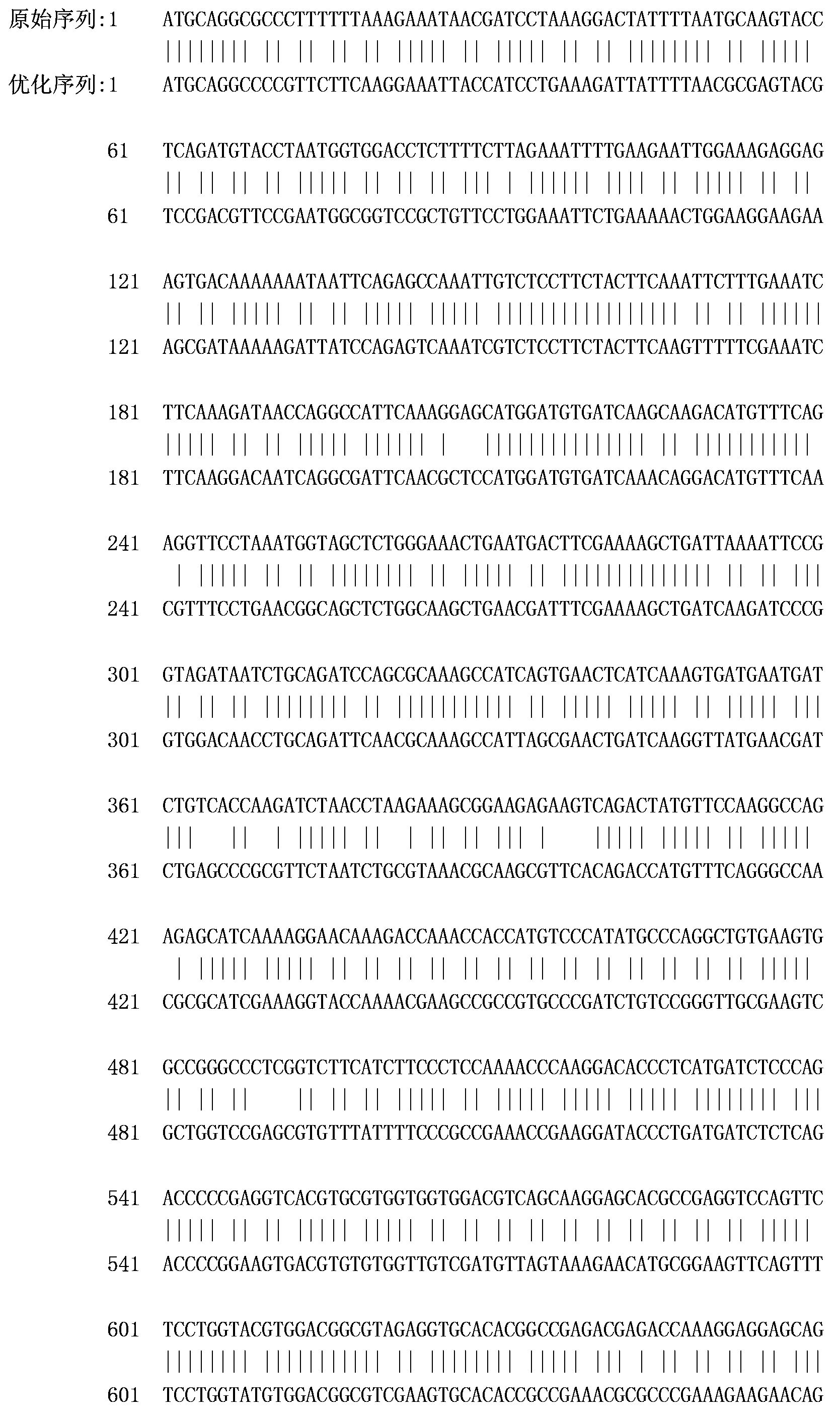
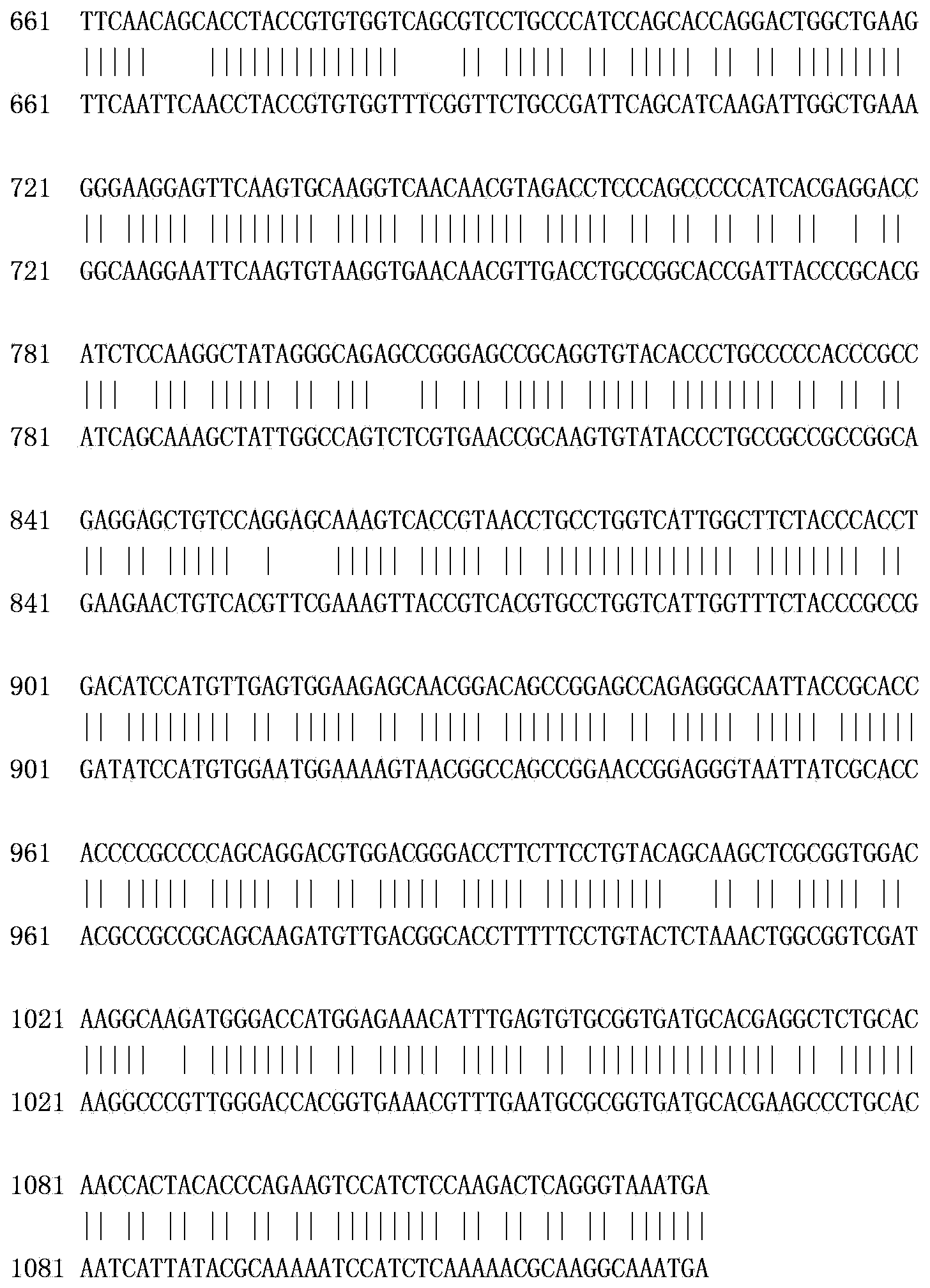
[0067] According to the cDNA sequence (GenBank accession number: NM_213948.1) and pig IgG Fc fragment (Sus scrofa IgG heavy chain) cDNA sequence (GenBank accession number: In the hinge region, CH2 region and CH3 region of NM_213828.1), these two genes are directly fused and codon optimized to obtain the gene of the recombinant porcine interferon gamma-Fc fusion protein of the present invention, as shown in SEQ ID No: 1 .
[0068] The following is the codon optimization of the recombinant porcine interferon γ-Fc fusion protein. The parameters before and after optimization are compared as follows:
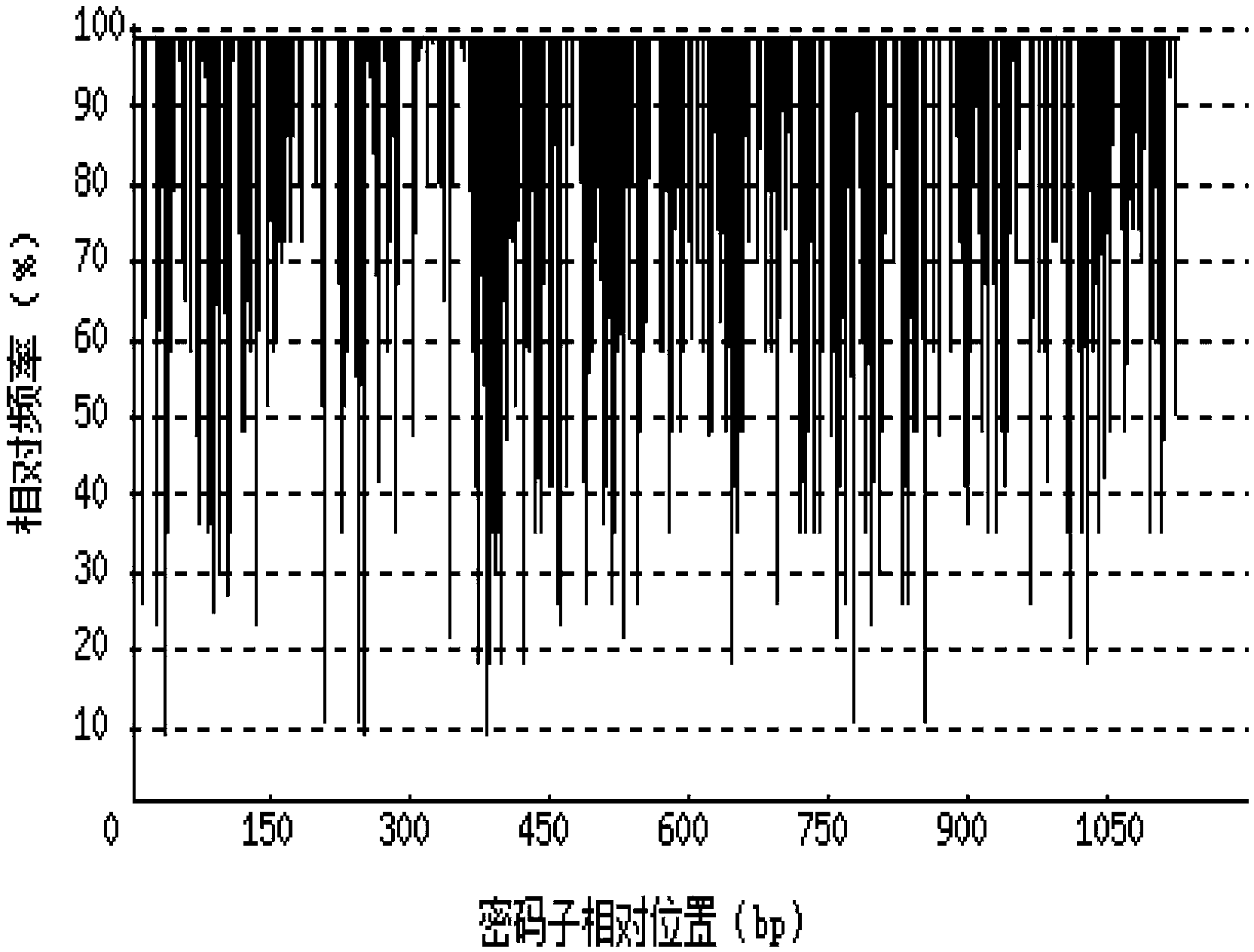
[0069] 1. Codon Adaptation Index (CAI)
[0070] Depend on Figure 2-a It can be seen that before codon optimization, the codon adaptation index (CAI) of the recombinant porcine interferon γ-Fc fusion protein gene in Escherichia coli was 0.64. Depend on Figure 2-b It can be seen ...
Embodiment 2
[0081] Embodiment 2: the expression plasmid construction of recombinant porcine interferon gamma-Fc fusion protein gene
[0082] The fragment synthesized from the optimized recombinant porcine interferon γ-Fc fusion protein gene (as shown in SEQ ID No: 1) was constructed into the pUC57 plasmid (provided by Nanjing GenScript Co., Ltd.) to obtain a long-term Save the plasmid and call it pUC57-pIFNγ-Fc plasmid. Using the pUC57-pIFNγ-Fc plasmid as a template, NdeI and XhoI restriction sites were introduced upstream and downstream, respectively, for PCR amplification. The primer sequences used are as follows:
[0083] Upstream primers:
[0084] P1: GGGAATTCCATATGCAGGCCCCGTTTCTTCAAGG
[0085] Downstream primers:
[0086] P2: CCGCTCGAGTCATTTGCCTTGCGTTTTTGAG
[0087] The total volume of the reaction was 50 μL, in which 2.5 μL of each primer was added at a concentration of 10 μmol / L, and 1 μL of dNTP at a concentration of 10 mmol / L was added. The DNA polymerase used was Phusion H...
Embodiment 3
[0089] Example 3 High-efficiency expression and identification of recombinant porcine interferon gamma-Fc fusion protein in Escherichia coli
[0090] Specific steps are as follows:
[0091] 1. Transform the pET21b-pIFNγ-Fc plasmid with correct sequence alignment in Example 2 into Escherichia coli BL21 (DE3) competent strain (purchased from Beijing Tiangen Biochemical Technology Co., Ltd.), at 37°C, on a plate containing ampicillin Incubate overnight.
[0092] 2. Pick 1-4 recombinant colonies containing the pET21b-pIFNγ-Fc plasmid the next day, insert them into LB culture medium (purchased from Amresco) containing 100 μg / mL ampicillin, and culture overnight at 37°C.
[0093] 3. Take 50 μL of the overnight culture in step 2, add 5 mL of LB culture solution containing 100 μg / mL ampicillin, and culture with shaking at 37°C.
[0094] 4. Measure the OD of the bacterial solution every 1 h after inoculation 600 value, to be OD 600 When =1.0, the expression was induced with 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com