The preparation method of theophylline sustained-release tablet
A technology for sustained-release tablets and theophylline, which is applied in the field of medicine, can solve the problems of poor reproducibility of sustained-release tablets, differences in release rate and dissolution, and achieve the effects of improving use value, stable quality, and avoiding toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
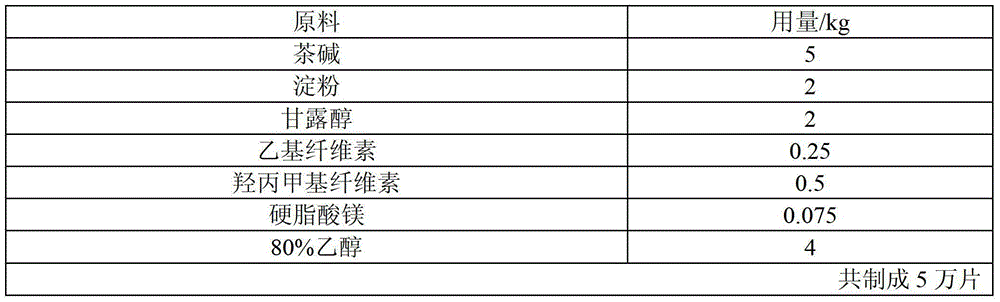
[0033] Table 1 The raw materials and proportions of theophylline sustained-release tablets (100mg)
[0034]
[0035] The preparation method is as follows:
[0036] (1) Add ethyl cellulose, hydroxypropyl methyl cellulose and 80% ethanol of recipe quantity in the wet mixing granulator, stir and shear at normal temperature for 30 minutes, and make a uniformly dispersed binder for subsequent use;
[0037] (2) Add theophylline, binding agent, starch and mannitol of recipe quantity in granulator again, make soft material;
[0038] (3) After replacing the granulator with a 16-mesh stainless steel mesh, use the granulator to granulate;
[0039] (4) The prepared wet granules are pumped into the fluidized bed, and the wet granules are vacuum pumped into the fluidized bed to dry to a moisture content of 4%, and then the granules are sized with a crushing and sizing machine, and magnesium stearate is added to mix evenly and then pressed The tablets are dried to obtain theophylline su...
Embodiment 2
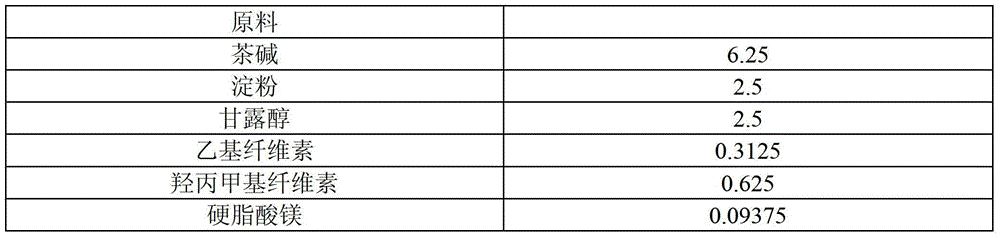
[0041] Table 2 Theophylline Sustained-release Tablets (125mg) Raw Materials and Proportioning
[0042]
[0043]
[0044] The preparation method is as follows:
[0045] (1) Add povidone K30, methyl cellulose and 80% ethanol of recipe quantity in the wet mixing granulator, stir and shear at normal temperature for 25 minutes, make the uniformly dispersed adhesive for subsequent use;
[0046] (2) Add theophylline, binding agent, starch and mannitol of recipe quantity in granulator again, make soft material;
[0047] (3) After replacing the granulator with 18-mesh stainless steel mesh, use the granulator to granulate;
[0048] (4) The prepared wet granules are pumped into the fluidized bed, and the wet granules are vacuum pumped into the fluidized bed to dry to 5% moisture, and then the granules are sized with a crushing and sizing machine, and magnesium stearate is added to mix evenly and then pressed The tablets are dried to obtain theophylline sustained-release tablets. ...
Embodiment 3
[0050] Table 3 The raw materials and proportions of theophylline sustained-release tablets (200mg)
[0051] raw material
Dosage / kg
Theophylline
5
starch
4
Ethyl cellulose
0.25
carbomer
0.1
Hypromellose
0.5
Magnesium stearate
0.01
stearic acid
0.075
80% ethanol
4
A total of 25,000 pieces were made
[0052] The preparation method is as follows:
[0053] (1) Add sodium alginate, hydroxypropyl methylcellulose and 80% ethanol in the wet mixing granulator, stir and shear at room temperature for 30 minutes, and make a uniformly dispersed adhesive for subsequent use;
[0054] (2) Add theophylline, binding agent, starch and mannitol of recipe quantity in granulator again, make soft material;
[0055] (3) After replacing the granulator with a 19-mesh stainless steel mesh, use the granulator to granulate;
[0056] (4) The prepared wet granules are pumped into the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com