Flt3 inhibitors for immune suppression
A technology of kinase inhibitors and uses, applied in the field of FLT3 inhibitors for immunosuppression, capable of solving problems such as immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0191] Treatment of DCs with FLT3 inhibitors and induction of apoptosis
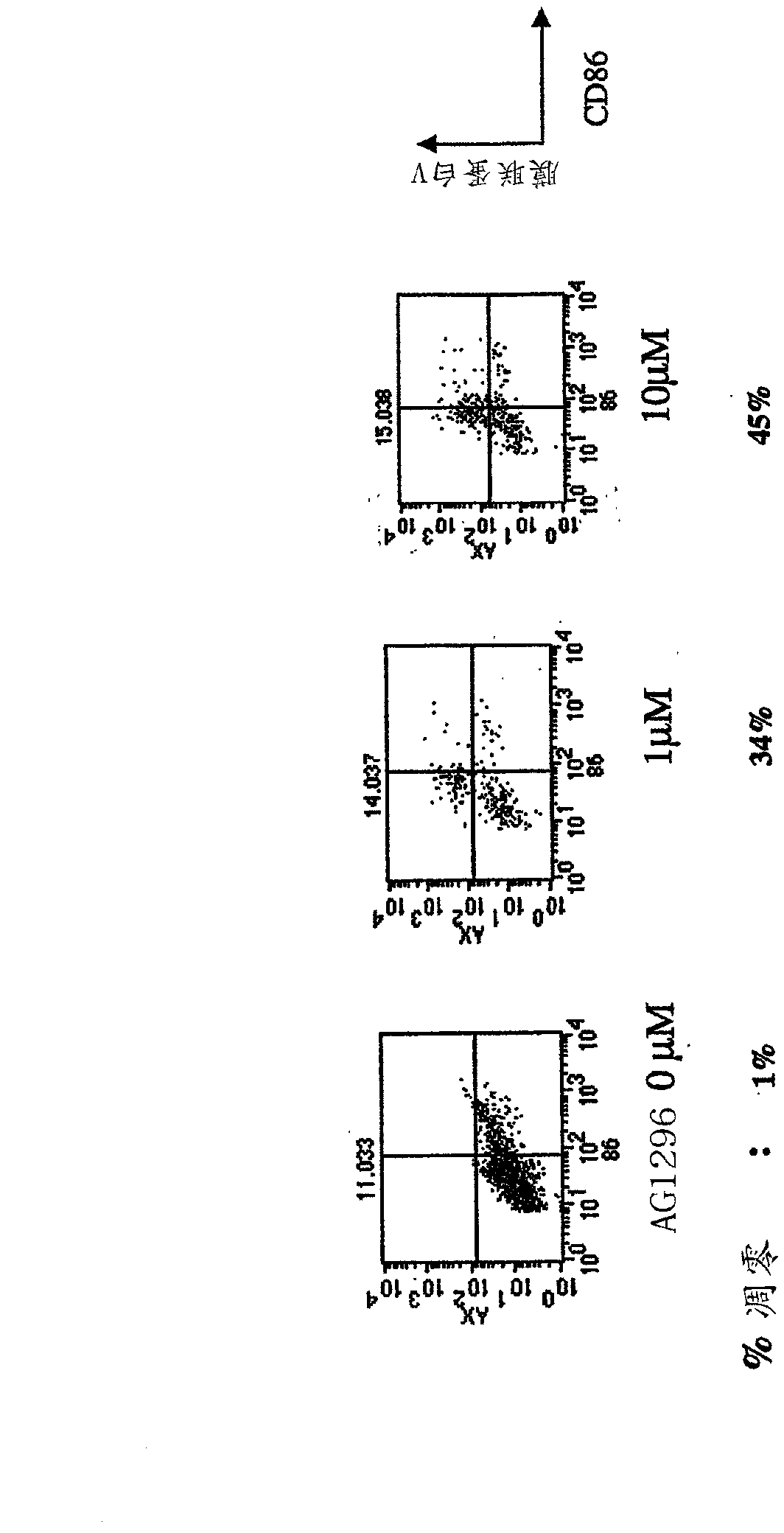
[0192] When DCs were exposed to FLT3 inhibitors, the survival and function of DCs were tested. In the presence of adenoviral transfectants-single colony-stimulating factor (GM-CSF) + / - FL, mature DCs were cultured in dose-response assays and Corresponding to CEP701, 5214 and AG1296, a decrease in viable and stimulated DCs was observed.
[0193] Bone marrow (BM) was flushed with PBS from mouse extremities, and red blood cells (RBCs) were lysed in hypotonic buffer. -CSF) (1000U / ml) or GM+FL (100ng / ml) at a concentration of 2x106cells / ml cultured in a culture dish, remove the non-adherent cells on the 2nd, 4th and 6th day and before adding fresh medium Wash the adherent cells, collect non-adherent mature DCs on the 8th day, and re-culture in the absence or presence of CEP-701 at a concentration of 5 or 50 molar. After 24 hours, the cells are stained for FACS analysis , combined with MHC class II expressio...
example 2
[0197] Inhibition of FLT3 reduces DC-basal proliferation of T cells. In these cases, DCs were generated from BALBc mice as described above, the cells were detached from untreated or treated with CEP701, and then cultured with C57BL / 6 splenocytes at the ratios indicated, After 3 days, join 3 H and measure proliferation as Figure 2a showed that CEP701 had a robust effect on reducing hyperplasia, Figure 2b In , BALB / c splenocytes replaced DCs cells to test the inhibition of CEP701 in the standard mixed lymphocyte reaction (MLR), and the figure also showed that the response was similarly inhibited in the presence of CEP701.
[0198] To determine whether T cell stimulation responses were suppressed by exposure to AG1296, two different T cell proliferation measurements were performed, Figure 2c Shown are results from co-incubating allogeneic mice in the presence (10 μM) or absence of inhibitors, mixed lymphocyte reactions in this standard, equal numbers of stimulator and respon...
example 3
[0200] Direct treatment of T cells without inhibiting T cell proliferation
[0201] Due to the influence of inhibitors on DCs cells rather than T cells themselves, it is not shown that Figure 2a and the resulting suppression of b, 1x10 6 The T cells were cultured on the culture dish covered with anti-CD3, then the T cells were stimulated with anti-CD28, after 2 days, adding 3 H and measured proliferation, as shown, had no effect on directly stimulated T cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com