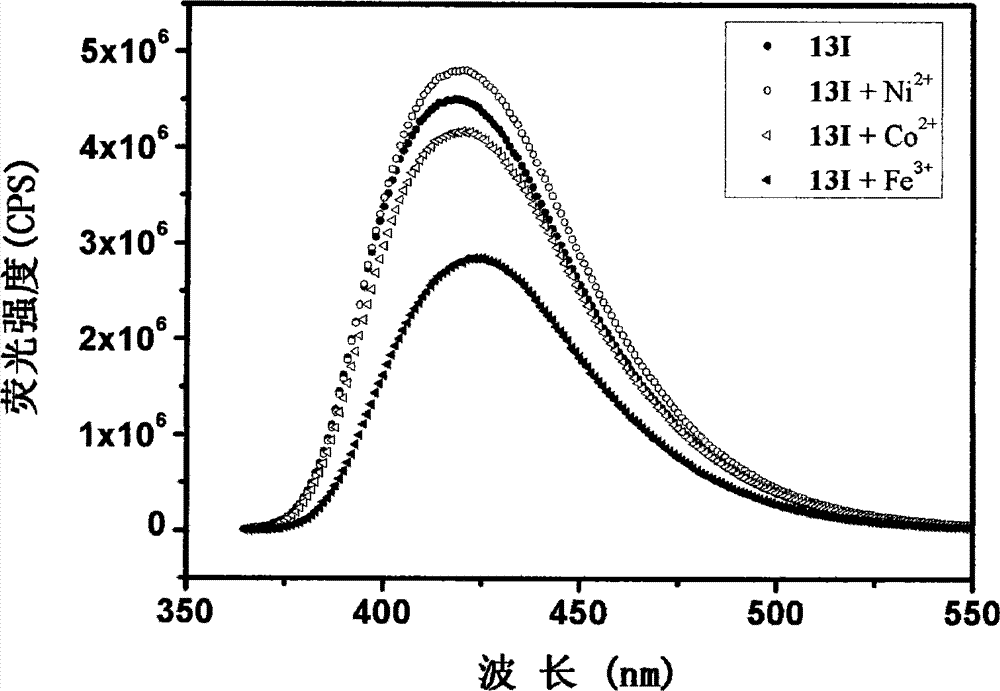
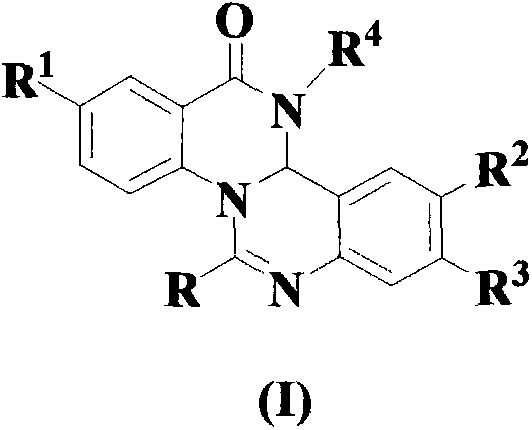
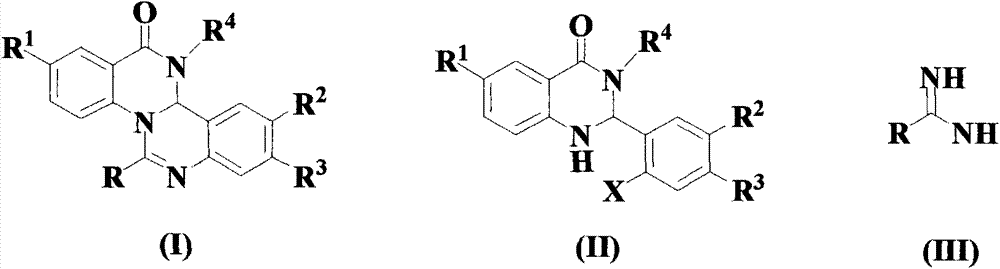
Quinazoline and quinazolinone compound, its synthesis method and application
A kind of quinazolinoquinazolinone, synthesis method technology, applied in quinazolinoquinazolinone compound and its synthesis, the field of nitrogen-containing heterocyclic compounds, can solve problems such as never involved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] (A) Preparation of compound (II)
[0067]
[0068] Add 10 mmol anthranilamide 1, 12 mmol 2-bromobenzaldehyde 2, 12 mmol citric acid and 10 ml ethanol into the reaction flask, and react under reflux and stirring at 80°C for 16 hours. After the reaction, use anhydrous MgSO 4 After drying, concentration under reduced pressure to remove ethanol, the residue was separated by 400 mesh silica gel column chromatography to obtain the target product (II) with a yield of 81.7%.
[0069] (B) Preparation of compound (I)
[0070]
[0071] In a reaction vessel equipped with a stirrer, a thermometer, and a feeding port, add 50 ml of solvent THF, and then add the above formula compounds (II), (III), Cu(acac) 2 And Cs 2 CO 3 , The molar ratio is 1:1:0.02:2, and the compound of formula (II) is 10 mmol. Replace with nitrogen for three times, and then react for 16 hours at 60°C with stirring under the protection of continuous nitrogen. After the reaction, the mixture was poured into ethyl acetat...
Embodiment 2
[0076] (A) Preparation of compound (II)
[0077]
[0078] Add 10mmol 2-amino-5-methylbenzamide 1, 12mmol 2-bromobenzaldehyde 2, 12mmol citric acid and 10ml ethanol into the reaction flask, and react under reflux and stirring at 80°C for 16 hours. After the reaction, use anhydrous MgSO 4 After drying, concentration under reduced pressure to remove ethanol, the residue was separated by 350 mesh silica gel column chromatography to obtain the target product (II) with a yield of 86.3%.
[0079] (B) Preparation of compound (I)
[0080]
[0081] In a reaction vessel equipped with a stirrer, a thermometer and a feeding port, add 50ml of solvent toluene, and then add the above formula compounds (II), (III), Cu(acac) 2 And Cs 2 CO 3 , The molar ratio is 1:2:0.05:3, and the compound of formula (II) is 10 mmol. Replace with nitrogen three times, and then react for 20 hours at 80°C with stirring under the protection of continuous nitrogen flow. After the reaction, the mixture was poured into et...
Embodiment 3
[0086] (A) Preparation of compound (II)
[0087]
[0088] Add 10mmol 2-amino-5-chlorobenzamide 1, 12mmol 2-bromobenzaldehyde 2, 12mmol citric acid and 10ml ethanol into the reaction flask, and react under reflux and stirring at 80°C for 16 hours. After the reaction, use anhydrous MgSO 4 After drying, concentration under reduced pressure to remove ethanol, the residue was separated by 400 mesh silica gel column chromatography to obtain the target product (II) with a yield of 82.5%.
[0089] (B) Preparation of compound (I)
[0090]
[0091] In a reaction vessel equipped with a stirrer, a thermometer and a feeding port, add 50ml of solvent dichloromethane, and then add the above formula compounds (II), (III), Cu(acac) 2 And Cs 2 CO 3 , The molar ratio is 1:3:0.08:4, wherein the compound of formula (II) is 10 mmol. Replace with nitrogen for three times, and then react for 24 hours at 90°C with stirring under the protection of continuous nitrogen. After the reaction, the mixture was pou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com