Thymopent aqueous solution preparation and use thereof
An aqueous solution and thymus technology, which is applied to the aqueous preparation of thymopentin, the application in medicine, and the field of pharmaceutical preparations, can solve the problems of inability to maintain the stability of the solution, shorten the time of stable storage, increase the cost, etc., and achieve the prevention of free calcium concentration. rapid decline, lower drug costs, lower shipping requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
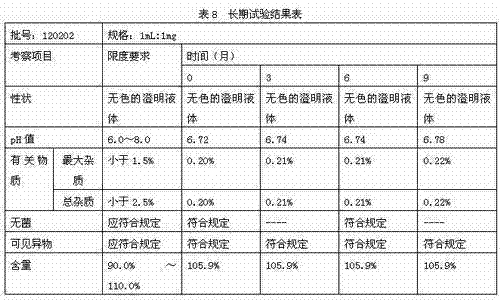
[0038] Accurately weigh 0.1g of thymopentin, 0.015g of sodium acetate, 1.485g of glacial acetic acid, 0.5g of calcium sodium edetate, and 0.3g of sodium chloride, dissolve them in water for injection, and finally dissolve them to 1000ml. The ampoules are divided into 1000 sticks. The final specification is 1ml / branch.
Embodiment 2
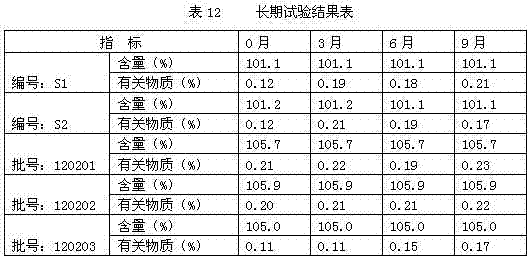
[0040] Accurately weigh 100g of thymopentin, 9.655g of sodium acetate, 990.345g of glacial acetic acid, 40g of calcium sodium ethylenediaminetetraacetate, and 200g of sodium chloride, dissolve them in water for injection, and finally dissolve them to 1000ml, and divide them into 2ml glass ampoules. Packed into 1000 pieces. The final specification is 1ml / branch.
Embodiment 3
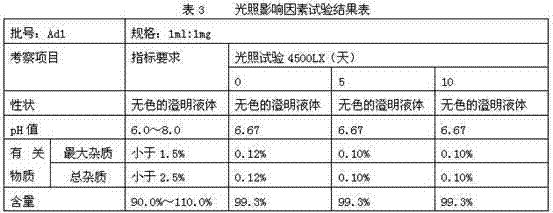
[0042] Accurately weigh 10g of thymopentin, 0.966g of sodium acetate, 99.034g of glacial acetic acid, 10g of calcium sodium edetate, and 20g of sodium chloride, dissolve them in water for injection, and finally dissolve them to 1000ml. Packed into 1000 pieces. The final specification is 1ml / branch.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com