A method for synthesizing 1-hydroxymethyl-3-hydrogen-2-oxaadamantane and derivatives thereof
A technology for adamantane and hydroxymethyl, which is applied in the field of synthesizing 1-hydroxymethyl-3-hydro-2-oxadamantane and derivatives thereof, and can solve problems such as inability to synthesize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
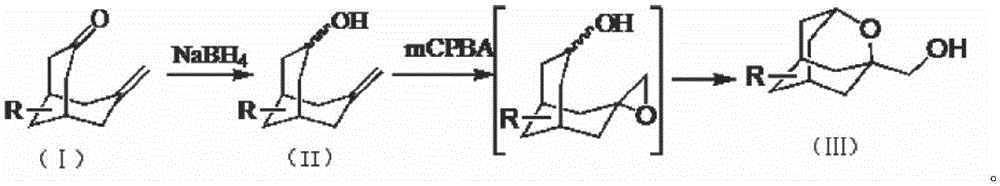
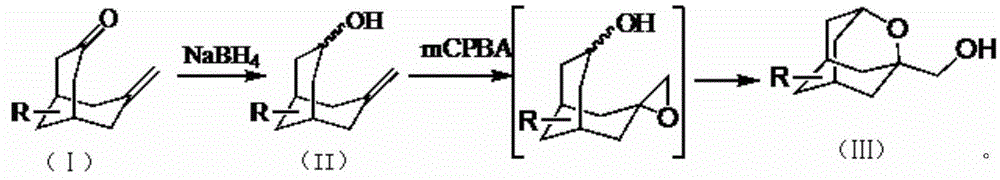
[0021] 1.1 Synthesis of 7-methylenebicyclo-[3.3.1]-nonan-3-ol
[0022]
[0023] Compound 7-methylenebicyclo-[3.3.1]-nonan-3-one (14g, 93mmol) was dissolved in THF / MeOH (100mL / 20mL), cooled to below 10°C in an ice-water bath, and slowly divided into 5 Sodium borohydride (6 g, 158 mmol) was added in batches, and after the addition was complete, the reaction was warmed to room temperature and stirred for 5 hours. Spin to dry, dilute with ethyl acetate / water (200mL / 100mL), separate the organic phase and dry with anhydrous sodium sulfate, and spin dry to obtain 14g of crude product, which is directly used in the next reaction.
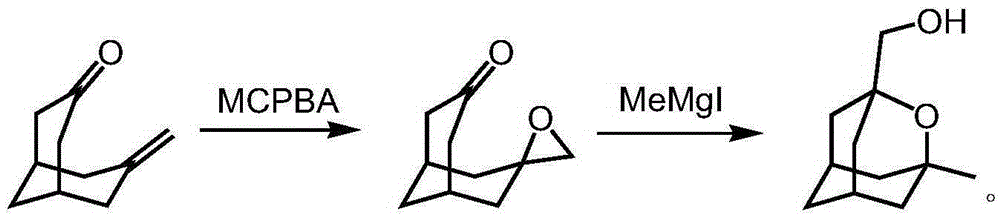
[0024] 1.2 Synthesis of 1-hydroxymethyl-2-oxaadamantane
[0025]
[0026] Compound 7-methylenebicyclo-[3.3.1]-nonan-3-ol was dissolved in dichloromethane (250mL), cooled to 0°C, and m-chloroperoxybenzoic acid (37.3g, 184 mmol), allowed to warm to room temperature after addition, and stirred overnight. TLC detected the completion of the reaction, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com