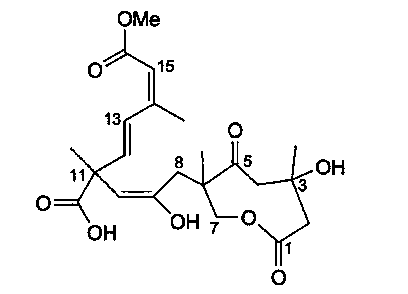
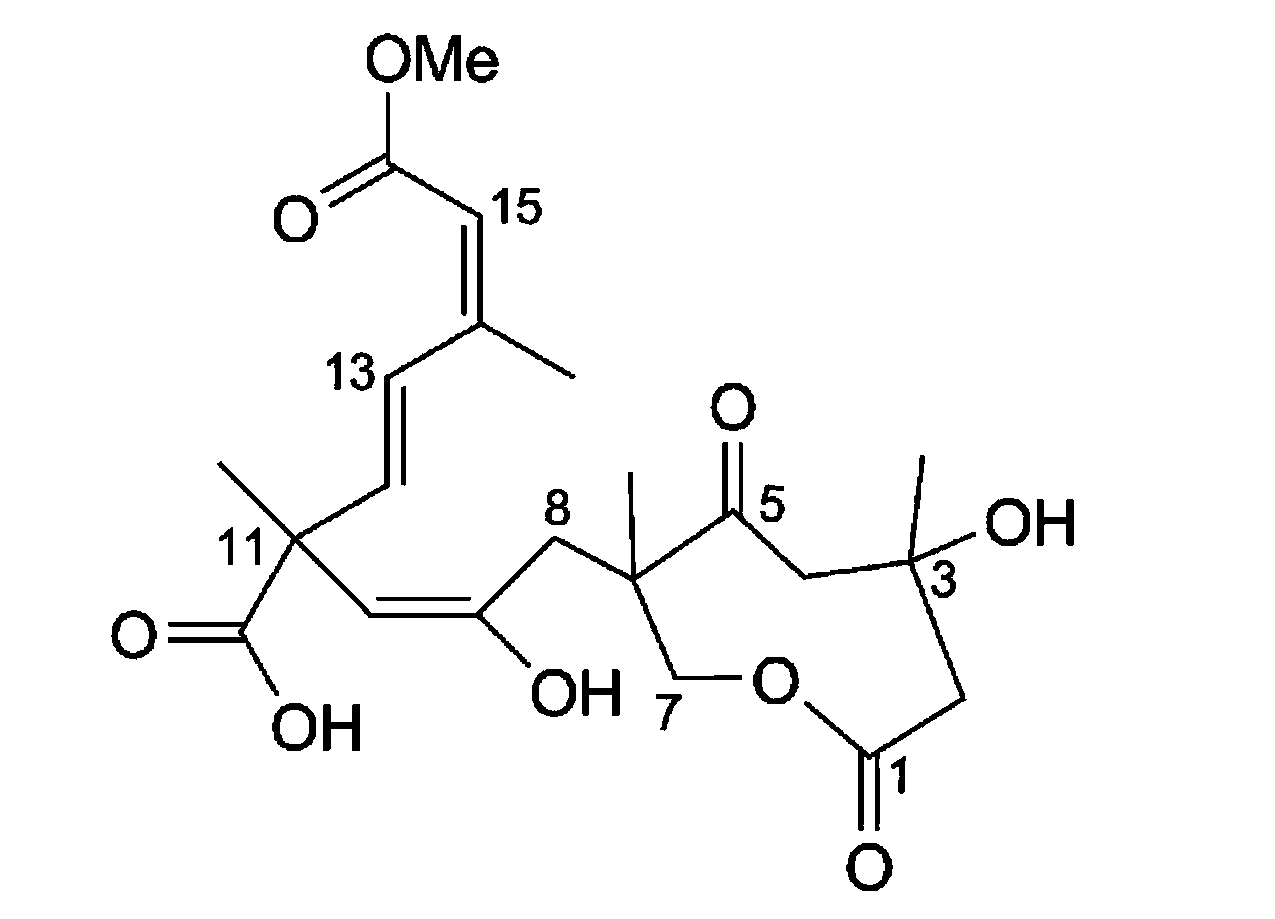
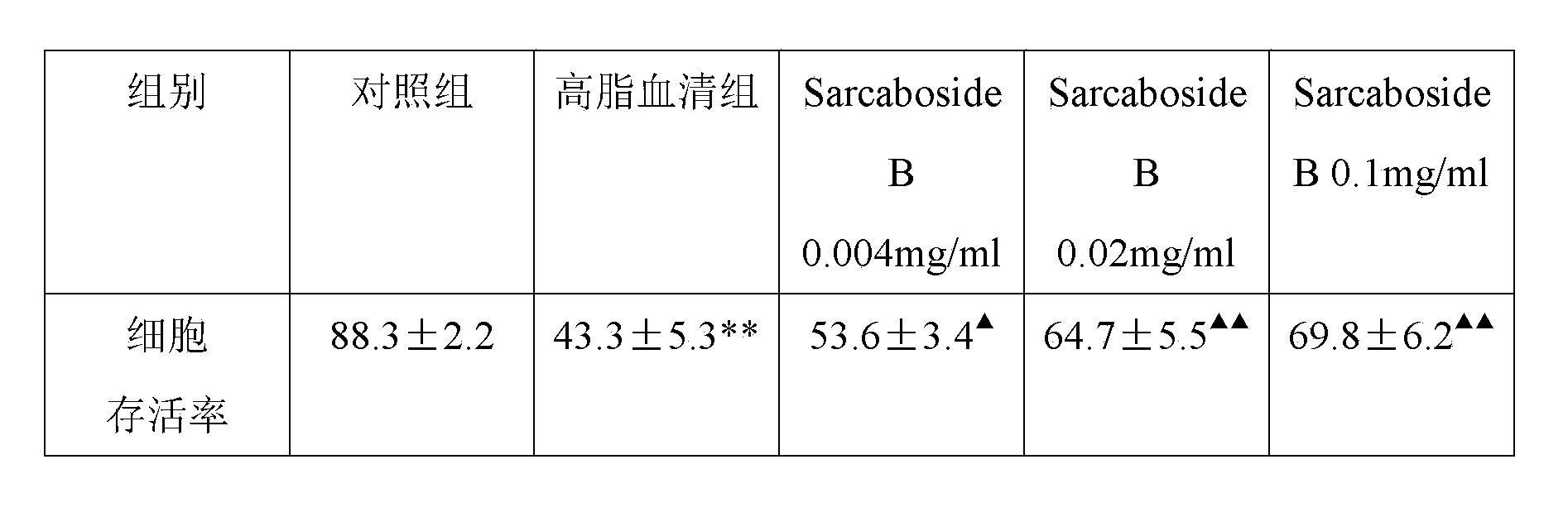
Application of Sarcaboside B in preparation of medicines for treating atherosclerosis
An atherosclerosis and drug technology, applied in the application field of Sarcaboside B in the treatment of atherosclerosis drugs, can solve the problems such as the cause and pathogenesis have not been fully elucidated, and there are no preventive measures and therapeutic drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: the preparation of compound Sarcaboside B tablet involved in the present invention:
[0013] Take 20 grams of compound Sarcaboside B, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tablet press.
Embodiment 2
[0014] Embodiment 2: the preparation of compound Sarcaboside B capsules involved in the present invention:
[0015] Get 20 grams of compound Sarcaboside B, add conventional auxiliary materials for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
[0016] The following pharmacodynamic experiments will further illustrate its drug activity.
[0017] The present invention reveals the effect of Sarcaboside B on atherosclerosis through cell level research and establishment of atherosclerosis model.
[0018] Oral and intravenous administration of Sarcaboside B in the present invention can significantly reduce plaque area / intima area (%), intima thickness (um) / media thickness (%) in atherosclerotic rats; Triesters, total cholesterol and low-density lipoprotein levels decreased, indicating that Sarcaboside B can treat atherosclerosis.
[0019] Materials and Methods
[0020] 1. Experimental materials:
[0021] 1.1 Animals and fee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com