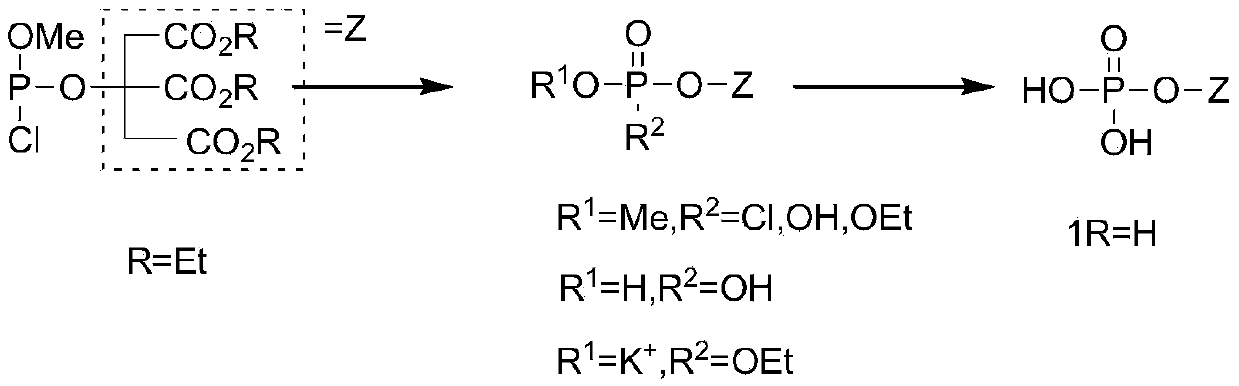
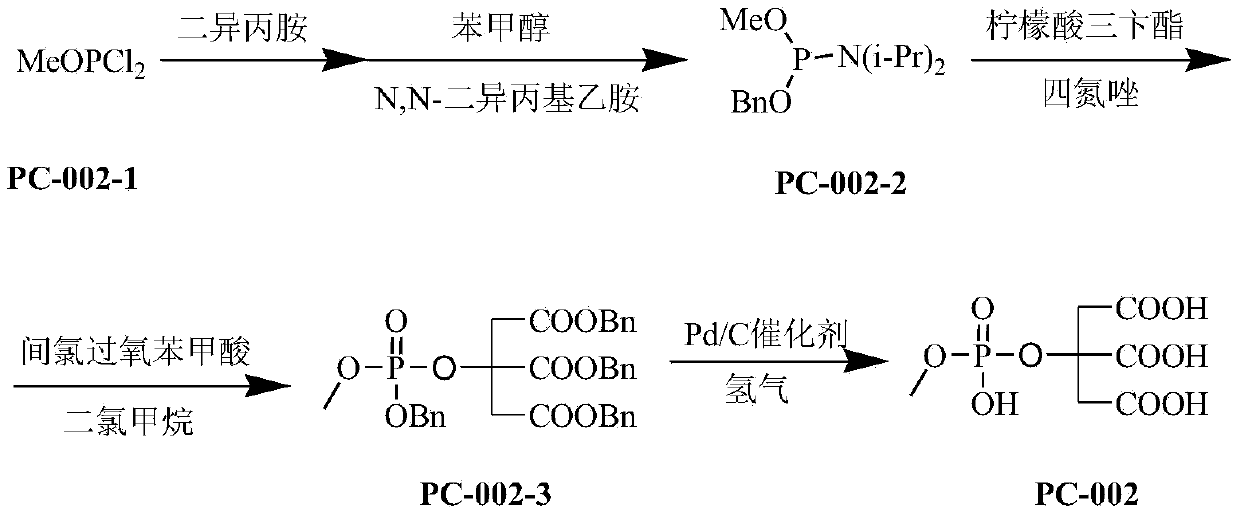
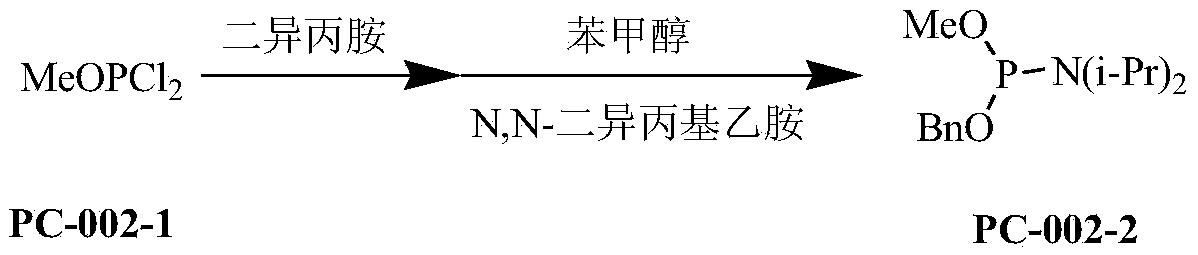
A kind of synthetic method of phosphoric acid citric acid derivative
A technology of citric acid phosphoric acid and derivatives, which can be used in drug combinations, phosphorus organic compounds, bone diseases, etc., can solve the problems of high price and unsuitable for large-scale synthesis, and achieve the effects of excellent quality, excellent product quality, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] First step response:
[0028]
[0029] Dissolve 100g of methoxyphosphorus dichloride in 280ml of anhydrous ether, cool to -20°C, the mass ratio of methoxyphosphorus dichloride to anhydrous ether is 1:2, and add dropwise to the solution containing 100g of diiso In the ether solution of propylamine, the mass ratio of diisopropanol to ether is 1:2, the mass ratio of methoxyphosphorous dichloride to diisopropanol is 1:1, react at room temperature for 2 hours, filter to remove the precipitate, and the filtrate Cool to -20°C, slowly add 50g N, N-diisopropylethylamine and 50g benzyl alcohol, the mass ratio of methoxyphosphorus dichloride to N, N-diisopropylethylamine is 1:0.5, and The mass ratio of benzyl alcohol is 1: 0.5, and reaction mixture rises to room temperature in 1 hour, stirs and reacts for 2 hours, filters the salt that reaction generates, and filter cake is washed with 200ml ether, and filtrate decompression evaporates solvent wherein, and with sherwood oil / T...
Embodiment 2
[0037] First step response:
[0038] Dissolve 1kg of methoxyphosphorus dichloride in 3.5L of anhydrous ether, cool to -20°C, the mass ratio of methoxyphosphorus dichloride to anhydrous ether is 1:2.5, and add dropwise to the solution containing 1.5kg In the ether solution of diisopropylamine, the mass ratio of diisopropylamine to ether is 1:2.5, the mass ratio of methoxyphosphorus dichloride to diisopropanol is 1:1.5, react at room temperature for 2 hours, filter to remove the precipitate, The filtrate was cooled to -20°C, slowly added 700g N, N-diisopropylethylamine and 700g benzyl alcohol, the mass ratio of methoxyphosphorus dichloride and N, N-diisopropylethylamine was 1:0.7, The mass ratio with benzyl alcohol is 1: 0.7, the reaction mixture is raised to room temperature in 1 hour, stirred and reacted for 2 hours, the salt generated by the reaction is filtered off, the filter cake is washed with 800ml ether, the filtrate is evaporated under reduced pressure to remove the so...
Embodiment 3
[0044] First step response:
[0045] Dissolve 500g of methoxyphosphorus dichloride in 2.1L of anhydrous ether, cool to -20°C, the mass ratio of methoxyphosphorus dichloride to anhydrous ether is 1:3, add dropwise to the solution containing 1kg of dichloride In the ether solution of isopropylamine, the mass ratio of diisopropylamine to ether is 1:3, the mass ratio of methoxyphosphorous dichloride to diisopropanol is 1:2, react at room temperature for 2 hours, filter to remove the precipitate, and the filtrate Cool to -20°C, slowly add 750g N, N-diisopropylethylamine and 750g benzyl alcohol, the mass ratio of methoxyphosphorus dichloride to N, N-diisopropylethylamine is 1: 1.5, and The mass ratio of benzyl alcohol is 1: 1.5, and reaction mixture rises to room temperature in 1 hour, stirs and reacts for 2 hours, filters the salt that reaction generates, and filter cake is washed with 800ml ether, and filtrate decompression evaporates solvent wherein, with sherwood oil / The crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com