Hard capsules with enteric film coating
A technology of hard capsules and hard capsule shells, applied in the directions of capsule delivery, carbon active ingredients, drug delivery, etc., can solve the problems of abnormal epithelial cell function, abnormal white blood cell function, increase liver toxicity, etc., to reduce absorption and help wound healing. , the effect of reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
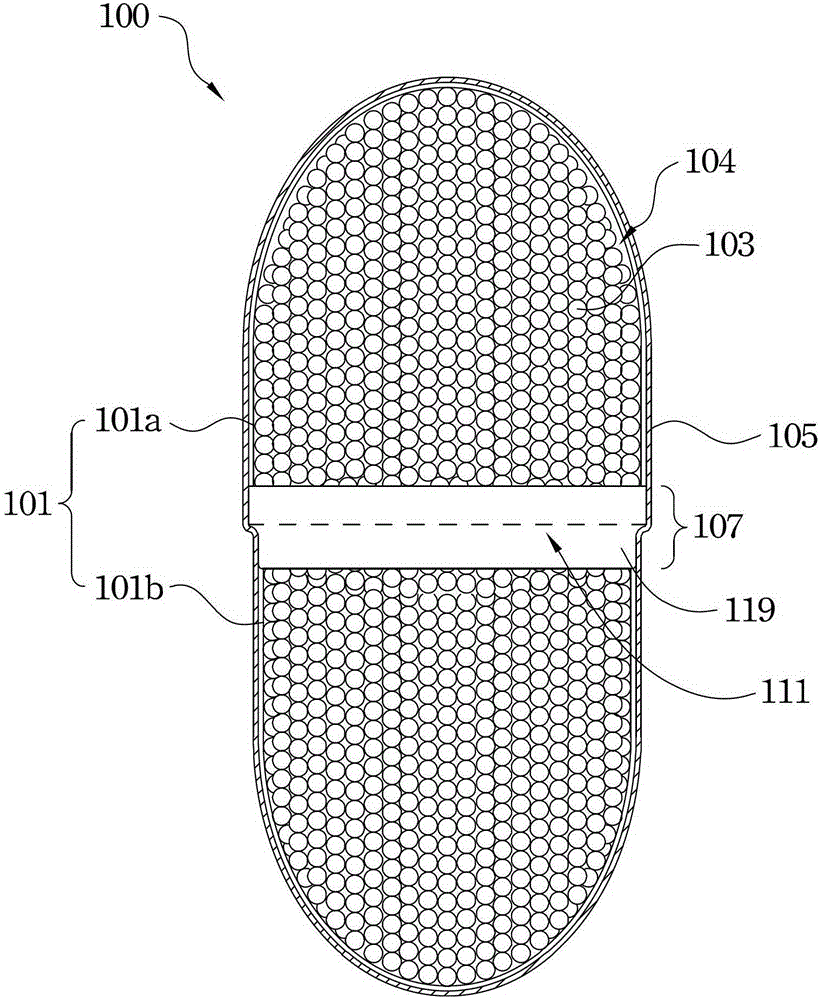
[0064] First, according to Table 1, take 50.0mg of EUDRAGITS100 ( GmbH & Co. KG, Darmstadt, Germany), 17.5 mg of triethyl citrate, 17.5 mg of propylene glycol, 2.5 mg of glyceryl monostearate, 1.0 mg of Tween80, mix well and dissolve in pure water to form Enteric film coating solution. Then, using a known sealing method, the thin strip made of hydroxypropyl methylcellulose is directly attached to the outside of the hard capsule shell and overlaps with the joining area of the capsule cap and the capsule body.
[0065] Then, soak the gelatin hard capsules filled with activated carbon (with spherical shape and irregular surface) in the enteric film coating solution and quickly take out and dry, or evenly spray the enteric film coating solution on the surface of the hard capsule and Dry to form hard capsules with an enteric film coating.
[0066] The obtained enteric-coated hard capsules were tested according to the evaluation methods described later, and the results are show...
Embodiment 2 to 8
[0068] With the preparation method of the hard capsule of the enteric film coating of embodiment 1, difference is that embodiment 2 to 8 changes the kind and the consumption of raw material in the enteric film coating composition, and its formula and test result are as shown in table 1, No further details here.
Embodiment 9 to 15
[0070] With the preparation method of the hard capsule of the enteric film coating of embodiment 1, difference is that embodiment 9 to 15 changes the kind of raw material in the enteric film coating composition, usage amount and the solvent of dissolving, and its formula and test result are as table 1, and will not be repeated here.
[0071]
[0072] assessment method
[0073] 1. Dissolution test:
[0074] First, to simulate the environment of the gastrointestinal tract, the enteric-coated hard capsules of Examples 1 to 15 were first added to 900 mL of a buffer solution with a pH of 1.2 to simulate the environment in the stomach. Stirring was continued for 120 minutes, wherein the buffer solution was prepared from potassium chloride, sodium chloride, dipotassium hydrogen phosphate and sodium hydroxide or any combination of the above. It should be added that the buffer solution is well known to those skilled in the art of the present invention, as long as the pH value and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com