Cefprozil dry suspension and preparation method thereof
A technology of cefprozil and dry suspension, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Complexity and other problems, to achieve the effect of good suspension effect, good dissolution rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
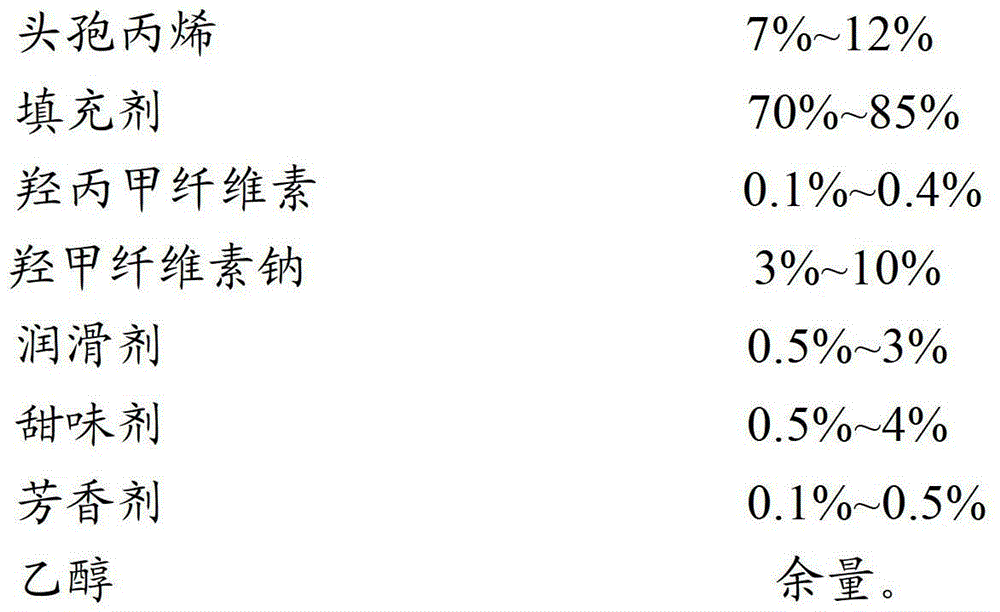
[0033] prescription:
[0034]
[0035] Preparation:
[0036] Take 1720g of sucrose, 4g of hypromellose K4M and 200g of hypromellose sodium in the mixer and mix evenly in the above prescription, then add 50g of micropowdered silica gel and 8g of 60% ethanol (8g is the quality of 60% ethanol, the following the same), put it in a mixing wet granulator and mix evenly, dry at low temperature, put it in a multi-directional mixer and mix evenly, add 63g of aspartame, 6g of strawberry essence, and 200g of cefprozil, mix evenly, and pack it separately to get cefprozil dry suspension.
Embodiment 2
[0038] prescription:
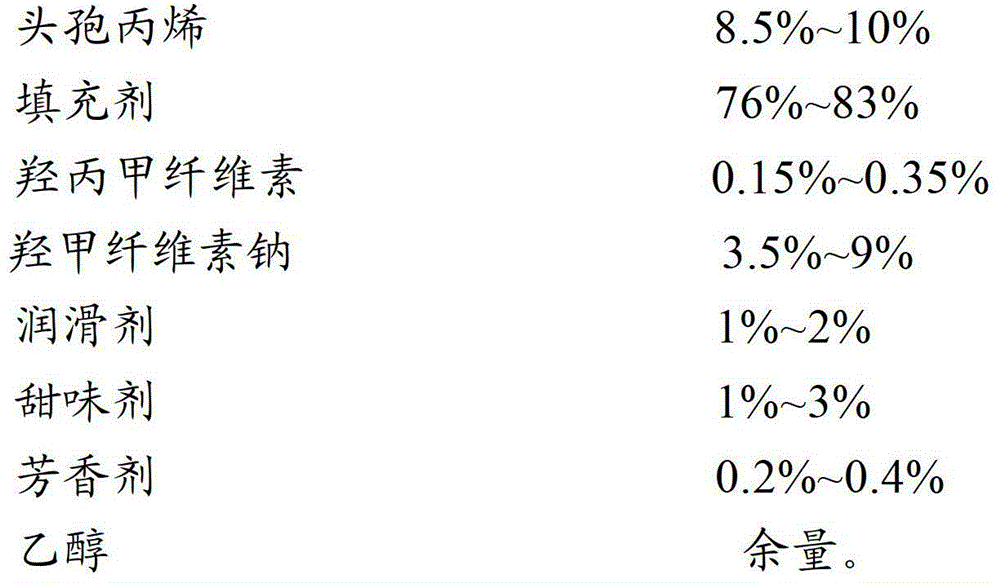
[0039]
[0040]
[0041] Preparation:
[0042] Take 2150g of sucrose, 10g of hypromellose K4M and 220g of hypromellose sodium in a mixer and mix them evenly in the mixer, then add 55g of micropowder silica gel and 10g of 60% ethanol, and mix them in a mixing wet granulator Uniformly, dry at low temperature, place in a multi-directional mixer to mix evenly, add 70g of aspartame, 10g of strawberry essence and 250g of cefprozil, mix evenly, and pack separately to obtain cefprozil dry suspension.
Embodiment 3
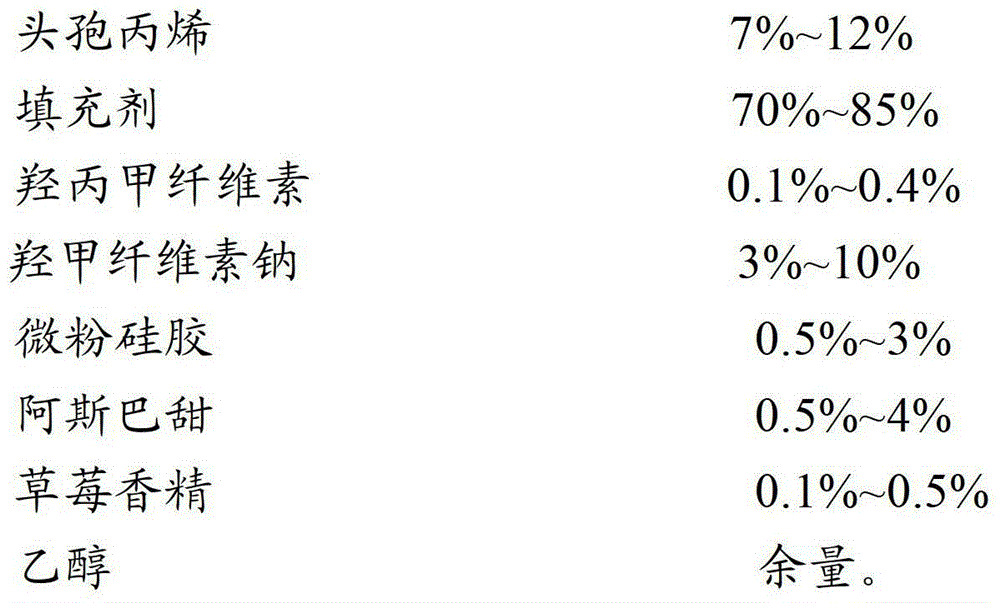
[0044] prescription:
[0045]
[0046] Preparation:
[0047] Take 4800g of sucrose, 13g of hypromellose K4M and 260g of hypromellose sodium in a mixer and mix them evenly in the mixer, then add 65g of micropowder silica gel and 24g of 60% ethanol, and mix them in a mixing wet granulator Uniformly, dry at low temperature, place in a multi-directional mixer to mix evenly, add 82g of aspartame, 13g of strawberry essence and 600g of cefprozil, mix evenly, and pack separately to obtain cefprozil dry suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com