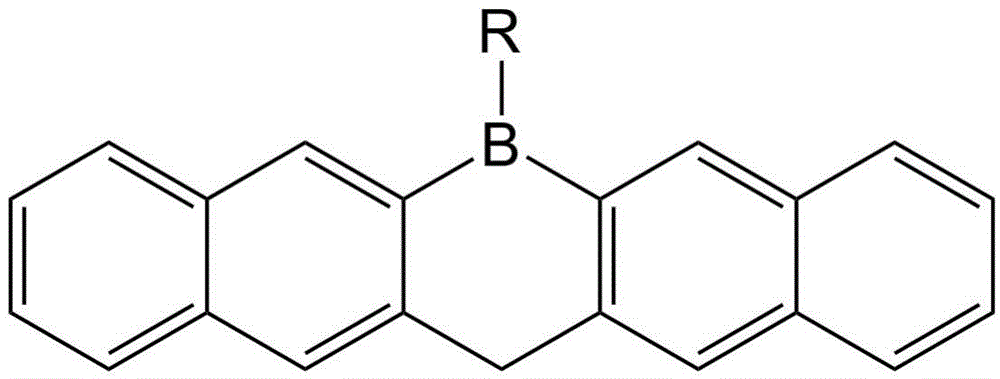
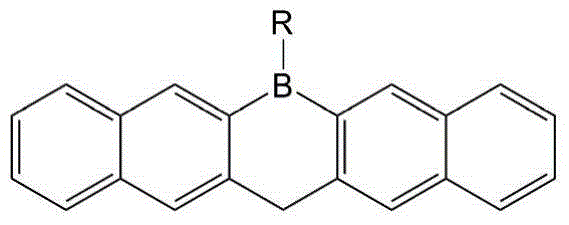
A kind of single boron heterocyclic organic electron transport material and preparation method thereof
A technology for transporting materials and organic electrons, which is applied in the field of single boron heterocyclic organic electron transporting materials and their preparation, and can solve the problems of low electron transport efficiency and low performance of organic light-emitting devices.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
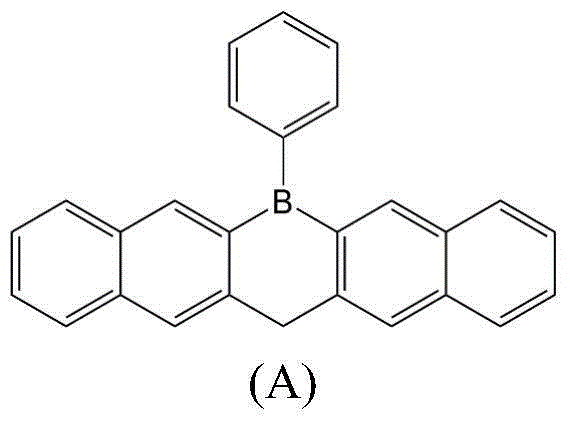
[0038] Under nitrogen protection, in a three-necked flask, dissolve 0.1mol 6-chloro-6,13-dihydronaphtho[2,3-b:2',3'-e]borane and 0.001mol palladium acetate in In 50mL of anhydrous tetrahydrofuran, cool to -78°C, add dropwise 60mL of 1M phenyl lithium hexane solution, dropwise, stir, continue to react for 24 hours, heat up to 25°C, add 200mL of chloroform and 100mL of water, separate liquid, organic The layer was dried over anhydrous sodium sulfate, filtered, concentrated, and the solid was purified by vacuum sublimation to obtain the organic electron transport material 6-phenyl-6,13-dihydronaphtho[2,3-b:2',3'- e] Bora ring (A), yield: 56%, melting point: >300°C, elemental analysis: C, 91.54; H, 5.41; B, 3.05, calculated value C, 91.51; H, 5.43; B, 3.06, Mass spectrometry: m / z: 354 (100.0%).
Embodiment 2
[0040] Under nitrogen protection, in a three-necked flask, dissolve 0.1mol 6-chloro-6,13-dihydronaphtho[2,3-b:2',3'-e]borane and 0.001mol palladium acetate in Into 50mL of anhydrous THF, cooled to -78°C, 60mL of 1M 2-methylphenyllithium hexane solution was added dropwise, the drop was completed, stirred, and the reaction was continued for 24 hours, the temperature was raised to 25°C, 200mL of chloroform and 100mL of water were added, Separate the liquid, dry the organic layer with anhydrous sodium sulfate, filter, concentrate, and purify the solid by vacuum sublimation to obtain the organic electron transport material 6-(2-methylphenyl)-6,13-dihydronaphtho[2, 3-b:2',3'-e]boracyclic ring (B), yield: 27%, melting point: >300°C, elemental analysis: C, 91.41; H, 5.70; B, 2.89, calculated value C, 91.32; H, 5.75; B, 2.94, mass spectrometry: m / z: 368 (100.0%).
Embodiment 3
[0042] Under nitrogen protection, in a three-necked flask, dissolve 0.1mol 6-chloro-6,13-dihydronaphtho[2,3-b:2',3'-e]borane and 0.001mol palladium acetate in Into 50 mL of anhydrous tetrahydrofuran, cool to -78°C, add dropwise 60 mL of 1M 3-methylphenyllithium hexane solution, dropwise, stir, continue to react for 24 hours, heat up to 25°C, add 200 mL of chloroform and 100 mL of water, Separate the liquid, dry the organic layer with anhydrous sodium sulfate, filter, concentrate, and purify the solid by vacuum sublimation to obtain the organic electron transport material 6-(3-methylphenyl)-6,13-dihydronaphtho[2, 3-b:2',3'-e]boracyclic ring (C), yield: 31%, melting point: >300℃, elemental analysis: C, 91.38; H, 5.72; B, 2.90, calculated value C, 91.32; H, 5.75; B, 2.94, mass spectrometry: m / z: 368 (100.0%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com