Applications of 1 type cysteinyl leukotriene receptor antagonist on preparation of medicament for treating the Alzheimer disease
A technology of cysteinyl leukotrienes, Alzheimer's disease, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] cCysLT 1 R activation leads to Aβ aggregation and memory impairment
[0020] experimental method
[0021] 1. Animals and groups
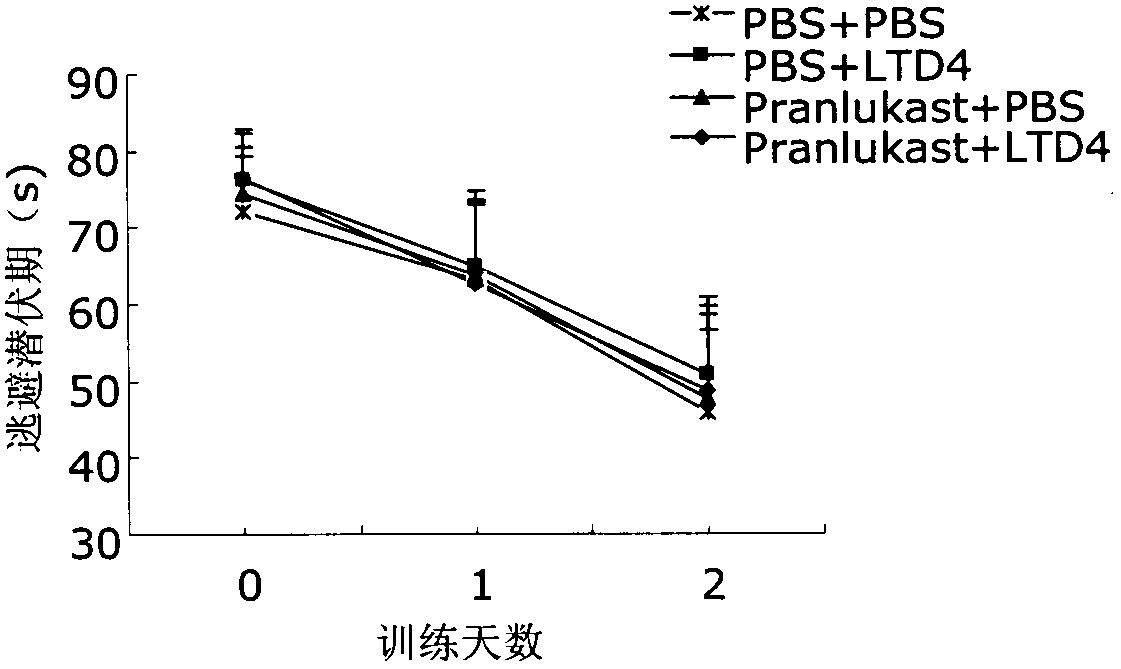
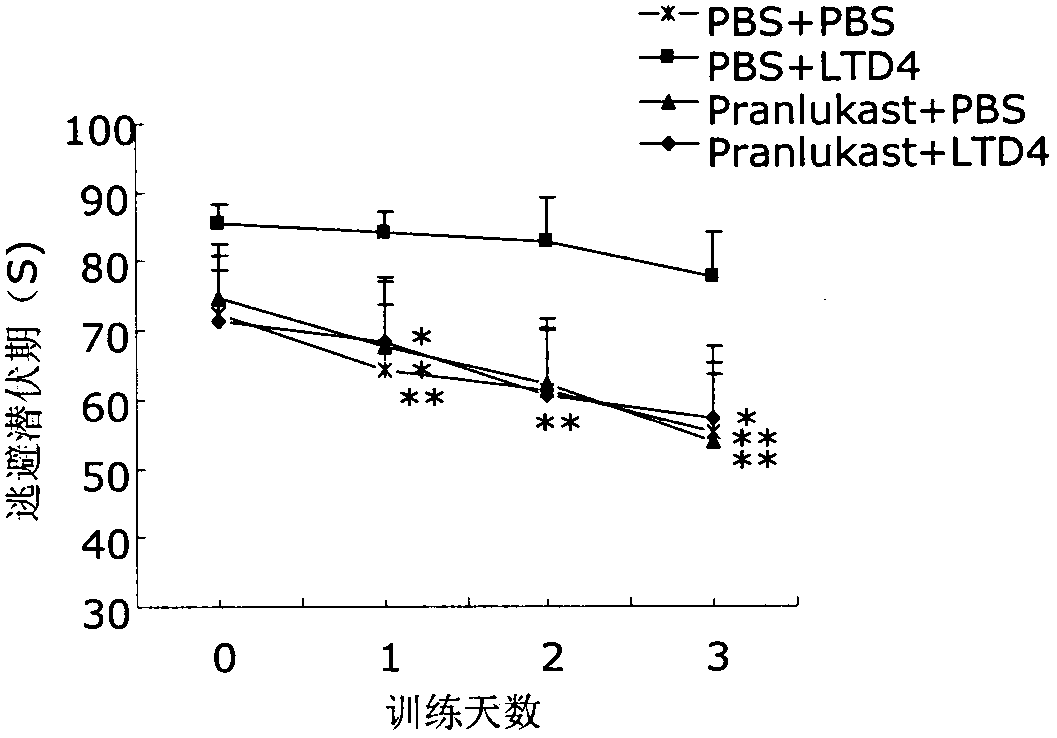
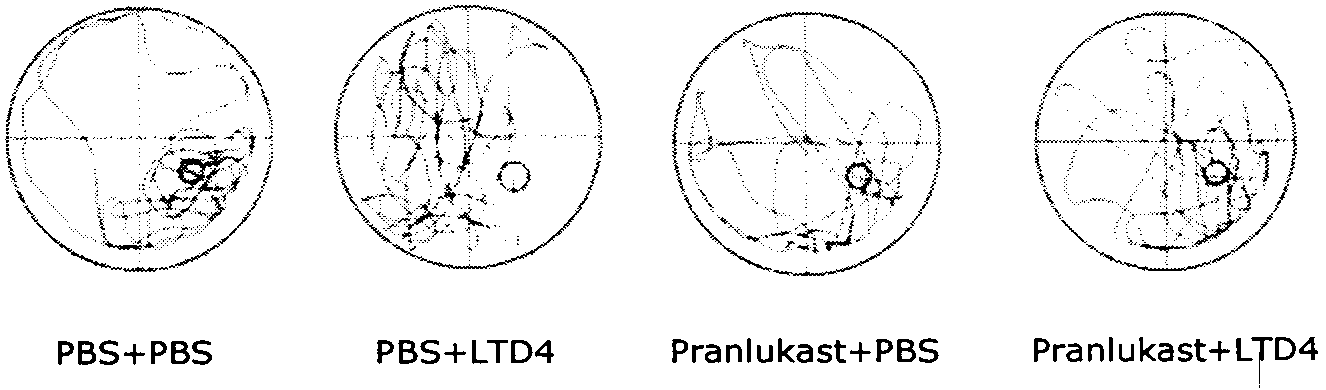
[0022] Take 40 clean-grade ICR male mice aged 8 weeks and weighing 22-25 g (provided by the Comparative Medicine Center of Yangzhou University, SCXK (Su) 2007-0001), and divide them into 4 groups, which are the normal control group (PBS+ PBS), cysteinyl leukotriene D4 group (LTD4+PBS), pranlukast group (pranlukast+PBS) and pranlukast plus cysteinyl leukotriene D4 group (pranlukast+LTD4), each Group 10. After bilateral intracerebroventricular injection, the memory function of the animals was evaluated by Morris water maze test after 72 hours. After the Morris water maze test, the animals were sacrificed, the brain was taken, the hippocampus and cortex were separated, and the hippocampus and cortex were detected by ELISA, Western blot and RT-PCR respectively. Aβ 40 , Aβ 42 and CysLT 1 R level.
[0023] 2. Morris water maze test
[002...
Embodiment 2
[0040] CysLT 1 R antagonist contralateral intracerebroventricular injection of Aβ 42 The source and structure of drugs that can improve learning and memory impairment in dementia-induced mice
[0041] Pranlukast, zafirlukast, and montelukast sodium are all raw materials produced by pharmaceutical companies, and their structures are as follows:
[0042]
[0043] pranlukast
[0044] (molecular formula: C 27 h 23 N 5 o 4 Molecular weight: 481.50)
[0045]
[0046] zafirlukast
[0047] (molecular formula: C 31 h 33 N 3 o 6 S molecular weight: 575.68)
[0048]
[0049] Montelukast
[0050] (molecular formula: C 35 h 36 ClNO 3 S molecular weight: 586.18)
[0051] experimental method
[0052] 1. Model establishment and administration
[0053] Get 8 weeks old, body weight is 120 clean level ICR mice of 22-25g, male and female (provided by the comparative medical center of Yangzhou University, SCXK (Su) 2007-0001), treated with 10% chloral hydrate (350mg / kg...
Embodiment 3
[0073] CysLT 1 R antagonists and their analogs against Aβ 42 The source and structure of drugs with protective effects on primary cultured mouse neurons
[0074] Pranlukast, zafirlukast and montelukast have the same structure as above.
[0075] The eight drugs of irast, alumilast, mitrolast, peripexil, cinalast, velukast, pyramolast, and ibudilast were purchased from different pharmaceutical or chemical companies , and their structure is as follows:
[0076]
[0077] iralukast
[0078] (molecular formula: C 38 h 37 f 3 o 8 S molecular weight: 710.76)
[0079]
[0080] ablukast
[0081] (molecular formula: C 28 h 34 o 8 Molecular weight: 498.56)
[0082]
[0083] Mitrodast
[0084] (molecular weight: C 13 h 12 N 2 o 2 S molecular formula: 260.31)
[0085]
[0086] Pobilukast
[0087] (molecular weight: C 26 h 34 o 5 S molecular formula: 458.61)
[0088]
[0089] Cinalukast
[0090] (molecular formula: C 23 h 28 N 2 o 3 S molecular...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com