Application of hexachlorophene in preparation of voltage-gated potassium channel agonist
A technology of agonist and hexachlorophene, applied in the field of application in the preparation of antiarrhythmic drugs and antiepileptic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 6
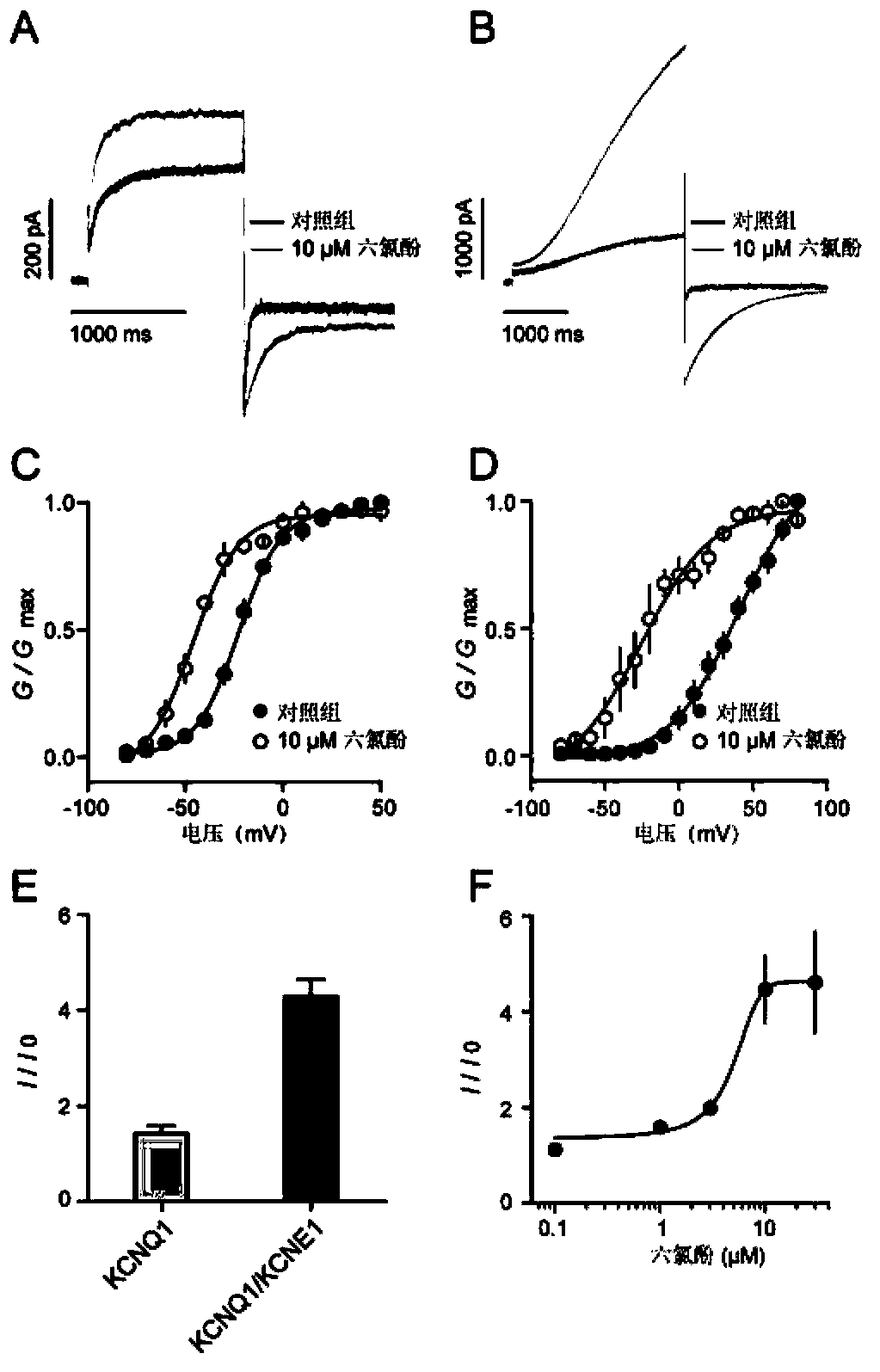
[0042] Example 1 Hexachlorophene Enhances KCNQ1 and KCNQ1 / KCNE1 Heteromeric Channel Current
[0043] 1. Cell Culture and Transfection
[0044] Chinese Hamster Ovary (Chinese Hamster Ovary, CHO) (Cell Bank of Chinese Academy of Sciences) culture solution formula: 50 / 50DMEM / F-12 (Gibco), adding 10% fetal bovine serum (Fetal bovine serum, FBS) (Gibco, Australia), 2 mM L-glutamine (Invitrogen).
[0045] Expression of KCNQ1 channel (KCNQ1 plasmid was donated by Professor Thomas J. Jenstsch of Zentrum für Molekulare Neurobiologie, Hamburg, the sequence was sequenced and verified with the NCBI database (National Center of Biotechnology Information, NCBI, USA) by using Megalign7 (DNAStar) sequence analysis and comparison software The number NM_000218.2 (KCNQ1) in the National Center for Biotechnology Information) has the same sequence): 24 hours before transfection, it was digested with trypsin (Sigma, China) and spread in a dish with a diameter of 35 mm. Transfection using Lipofec...
Embodiment 2 6
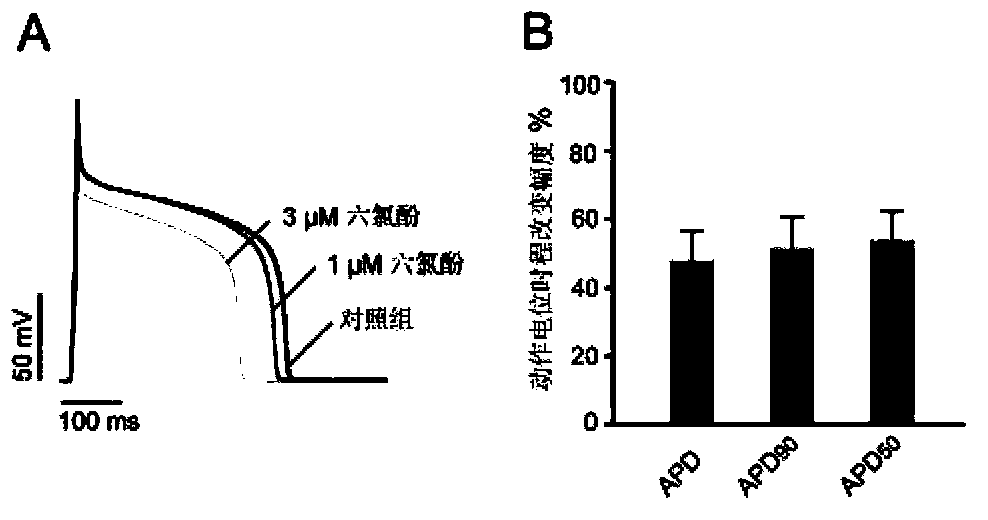
[0058] Embodiment 2 Hexachlorophene shortens the action potential duration of cardiomyocytes
[0059] In normal cardiomyocytes, KCNQ1 / KCNE1 heteromeric channels constitute outward I Ks The current participates in the second phase / end of the plateau phase and the third phase / rapid repolarization of the action potential of cardiomyocytes, and affects the time course of the action potential of the cardiomyocytes. However, since there is no unified understanding of the formation ratio of KCNQ1 and KCNE1 in vivo, in order to investigate the hexachloride that can enhance the KCNQ1 / KCNE1 heteromeric channel expressed heterologously in Chinese hamster ovary cells at a mass ratio of 50 / 50 Whether phenol can enhance the KCNQ1 / KCNE1 heteromeric channel in the body, to investigate whether hexachlorophene can affect the whole cardiomyocyte action potential time course by enhancing the KCNQ1 / KCNE1 heteromeric channel, the inventors of the present invention will 1 μM and 3 μM hexachlorophen...
Embodiment 3 6
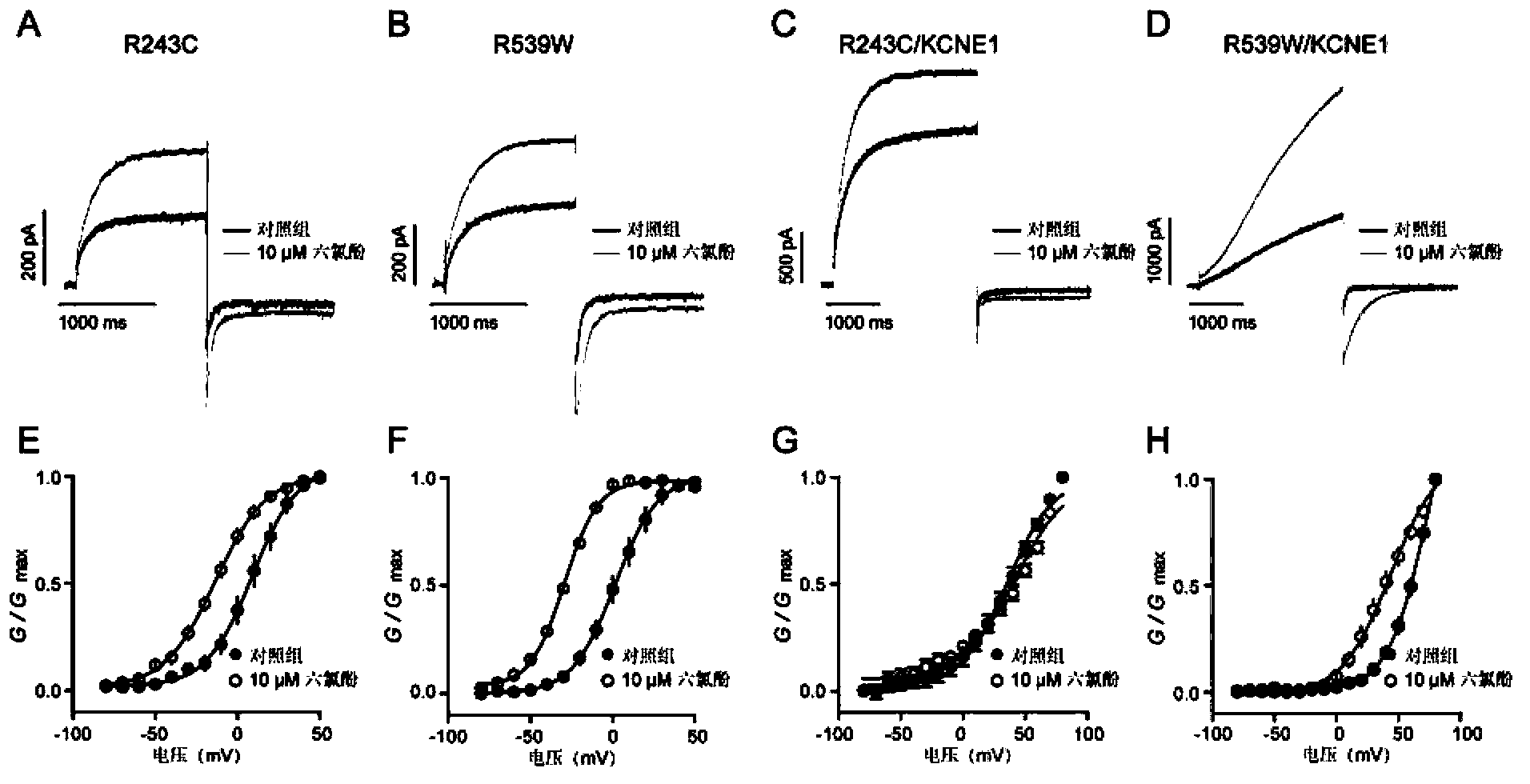
[0063] Example 3 Hexachlorophene rescues type I long QT syndrome mutation channel function
[0064] In the formation of a complete action potential in cardiomyocytes, different ion channels are involved, including KCNQ1, HERG, SCN5A (Bianchi, L. et al. Am J Physiol Heart Circ Physiol 2000, 279(6), H3003-11. and Hedley, P.L. Hum Mutat 2009, 30(11), 1486-511.), KCNE1, KCNE2, etc., the abnormal function of these ion channels will lead to long QT syndrome. However, among the long QT syndromes caused by these mutations, more than 50% of congenital long QT syndromes and more than 90% of acquired long QT syndromes are attenuated by KCNQ1 mutations, It is a type of long QT syndrome.
[0065] In vivo and in vitro experiments have shown that hexachlorophene, as a KCNQ1 and KCNQ1 / KCNE1 agonist, can enhance the channel current, shift the conductance voltage curve to the left, shorten the action potential duration and other pharmacological effects. In order to detect whether hexachloroph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com