Hepatocyte growth factor mimics as therapeutic agents
A hepatocyte growth factor and simulant technology, which is applied in the field of hepatocyte growth factor simulants as therapeutic agents, can solve the problems of high cost, preventing blood-brain barrier penetration rate, and limiting widespread use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
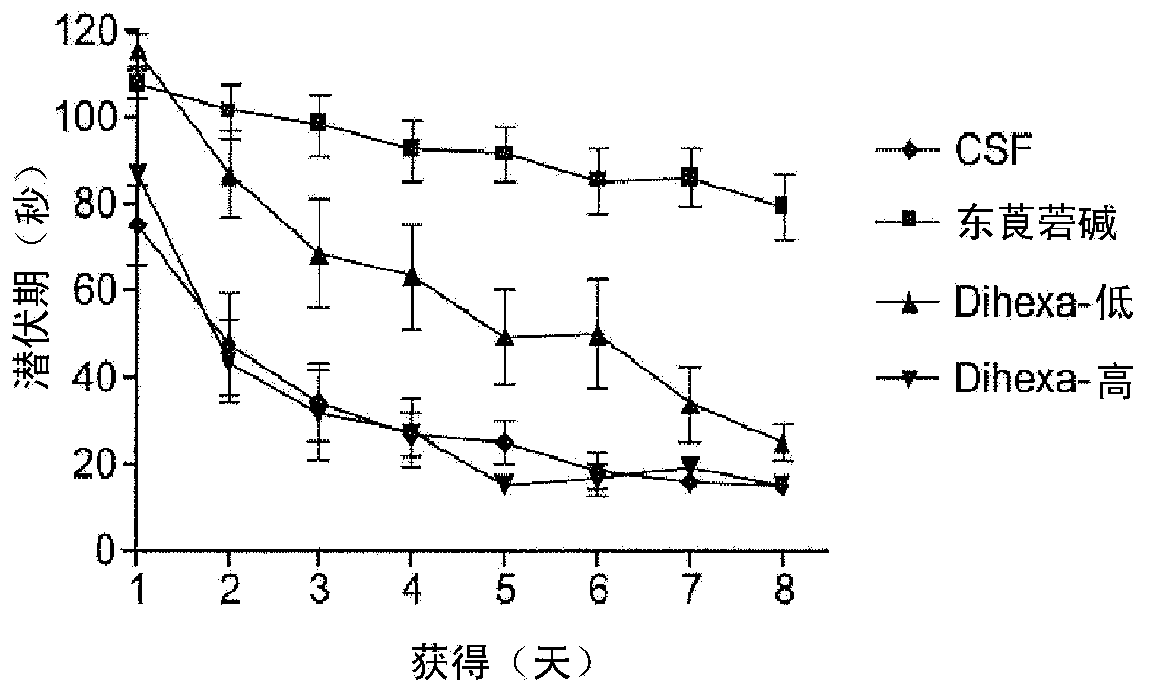
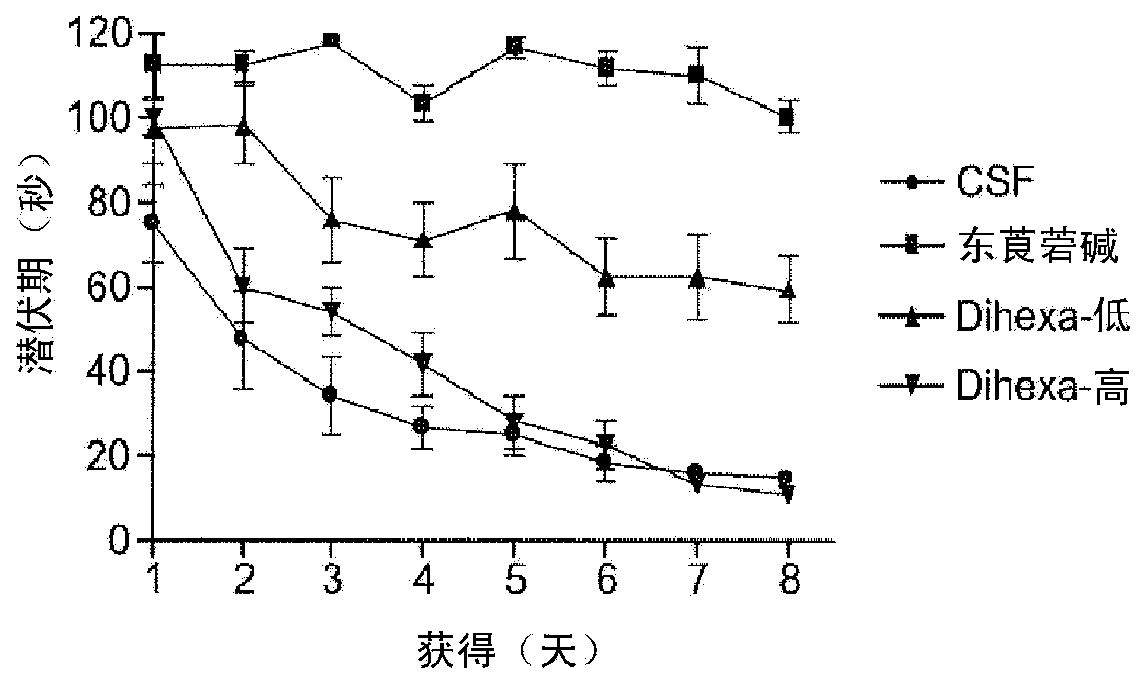
[0172] Embodiment 1Dihexa and Nle 1 - AnglV regulates synaptogenesis.
[0173] Nle of AngIV has been found before 1 - Tetrapeptide of AngIV analog (Nle 1 -YIH) and tripeptide (Nle 1 -YI) fragments are the minimally active fragments capable of overcoming scopolamine-induced cognitive impairment in a spatial learning task. Using the tripeptide as a novel template, additional active analogs with improved metabolic capacity, blood-brain barrier permeability, and oral efficacy were synthesized. In this example, we demonstrate the novel, orally active, angiotensin IV analog, Dihexa. Materials and Methods
[0174] Animals and Surgery. Male Sprague-Dawley rats (Taconic origin) weighing 390-450 g had free access to food and water (Harland Tekland F6 Rat Chow, Madison, WI) except when food was removed the night before surgery. Each animal was anesthetized with ketamine hydrochloride and xylazine (100 and 2 mg / kg intramuscularly, respectively; Phoenix Scientific; St. Joseph, MO, a...
Embodiment 2
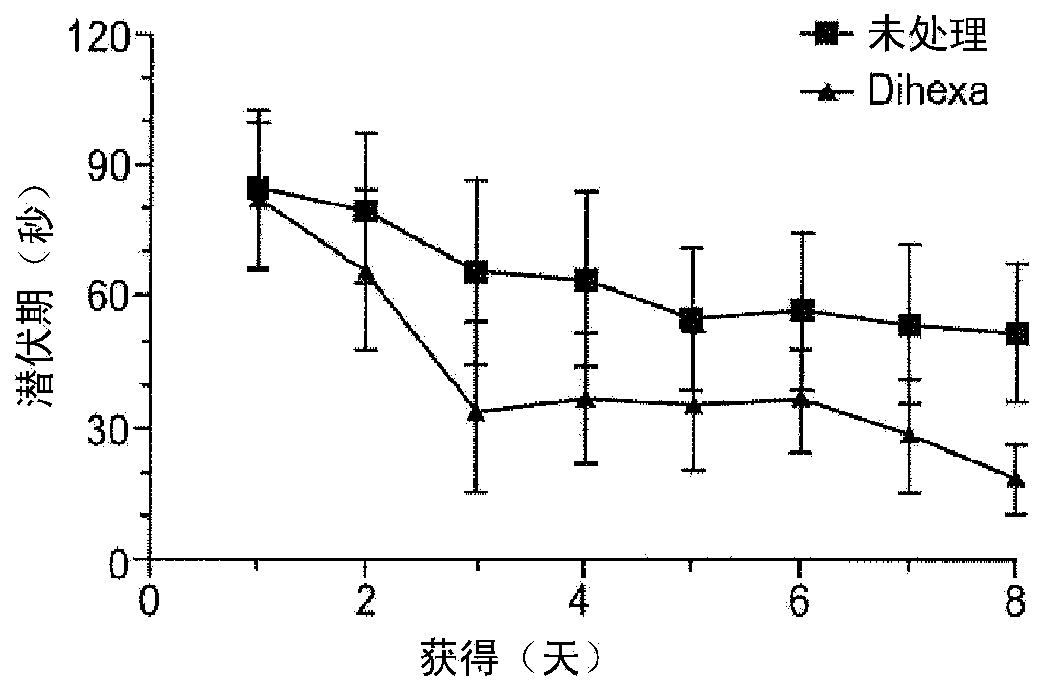
[0204] Example 2. Target of AngIV analogs is liver growth factor
[0205] This example shows that the novel angiotensin IV ligand Dihexa and its parent molecule Nle 1 - AngIV acts through the HGF / c-Met receptor system.
[0206] Materials and Methods
[0207] Animals and Surgery
[0208] Male Sprague-Dawley rats (Taconic origin) weighing 390-450 g had free access to food and water (Harland Tekland F6 Rat Chow, Madison, WI) except when food was removed the night before surgery. Each animal was anesthetized with ketamine hydrochloride and xylazine (100 and 2 mg / kg intramuscularly, respectively; Phoenix Scientific; St. Joseph, MO, and Moby; Shawnee, KS). Using the flat skull calibration function, a lateral ventricle (icv) guide catheter (PE-60, Clay Adams; Parsippany, NY) was placed stereotaxically (Model 900, David Kopf Instruments; Tujunga, CA) 1.0 mm posterior and anterior to bregma in the right hemisphere. 1.5 mm lateral to the bregma (see Wright et al.1985). The guide tu...
Embodiment 3
[0259] Example 3: Development of Angiotensin IV Analogs as Hepatocyte Growth Factor / Met Modifiers
[0260] The 6-AH family of angiotensin IV analogues [D-Nle-X-Ile-NH-(CH 2 ) 5 -CONH 2 ; where X = various amino acids] can directly bind to hepatocyte growth factor (HGF) and inhibit the ability of HGF to form functional dimers. Metabolically stable 6-AH family member D-Nle-Tyr-Ile-NH-(CH 2 ) 5 -CONH 2 in the blood 1 / 2 time of 80 min, compared to the parent compound Norleual (Nle-Tyr-Leu-ψ-(CH 2 -NH 2 ) 3-4 -His-Pro-Phe, SEQ ID NO:1) in blood t 1 / 2 The time is 3 The interaction of the H-hinge domain peptides results in a reduced ability of HGF to activate its receptor Met. This interference translates into inhibition of HGF-dependent signaling, proliferation and dispersion of concentrations in multiple cell types into the low picomolar range. We also noticed a significant correlation between 6-AH family members blocking HGF dimerization and inhibiting cellular activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com