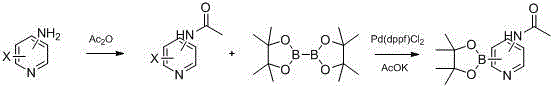
Preparation method of commonly used acetamidopyridine boronic acid pinacol ester
A technology of acetylaminopyridine boric acid and halogenated acetylaminopyridine, which is applied in the field of preparation of acetamidopyridine boric acid pinacol ester, and achieves the effects of convenient industrial production, simple preparation method and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A general method for preparing acetylaminopyridine borate pinacol ester, taking the synthesis of 2-acetylaminopyridine-4-boronic acid pinacol ester as an example:
[0023] The preparation of the first step 2-acetylamino-4-bromopyridine:
[0024] Add 45.84g (0.265mol) of 2-amino-4-bromopyridine to a 1L four-necked flask equipped with magnetic stirring and a thermometer, add 500mL of dichloromethane and stir at room temperature to dissolve, slowly add 40.52g (0.397mol) Acetic anhydride, react for 2 to 5 hours after dropping, control in TLC until the reaction is complete, distill under reduced pressure, dissolve the residue with ethyl acetate, wash twice with 200mL saturated sodium bicarbonate, and evaporate to dryness to obtain 2-acetamido-4- Bromopyridine 55.91g, yield 98.14%.
[0025] The preparation of second step 2-acetylaminopyridine-4-boronic acid pinacol ester:
[0026] Add 2-acetamido-4-bromopyridine 55.91g (0.26mol), bis(pinacolate) diboron 66.02 to a 1L four-n...
Embodiment 2
[0028] A general method for preparing acetylaminopyridine borate pinacol ester, taking the synthesis of 2-acetylaminopyridine-5-boronic acid pinacol ester as an example:
[0029] The preparation of the first step 2-acetylamino-5-bromopyridine:
[0030] Add 46.04g (0.267mol) of 2-amino-5-bromopyridine to a 1L four-necked flask equipped with a magnetic stirrer and a thermometer, add 500mL of dichloromethane and stir at room temperature to dissolve, slowly add 53.80g (0.527mol) Acetic anhydride, reacted for 2-5 hours after dropping, controlled by TLC until the reaction was completed, distilled under reduced pressure, dissolved the residue with ethyl acetate, washed twice with 200mL saturated sodium bicarbonate, and evaporated to dryness to obtain 2-acetamido-5- Bromopyridine 56.41g, yield 99.04%.
[0031] The preparation of second step 2-acetylaminopyridine-5-boronic acid pinacol ester:
[0032] Add 2-acetamido-5-bromopyridine 56.41g (0.264mol), bis(pinacolate) diboron 66.02 to...
Embodiment 3
[0034] A general method for preparing acetylaminopyridine boric acid pinacol ester, taking the synthesis of 3-acetylaminopyridine-5-boronic acid pinacol ester as an example:
[0035] The preparation of the first step 3-acetylamino-5-bromopyridine:
[0036] Add 45.04g (0.259mol) of 3-amino-5-bromopyridine to a 1L four-necked flask equipped with magnetic stirring and a thermometer, add 500mL of dichloromethane and stir at room temperature to dissolve, slowly add 26.44g (0.259mol) Acetic anhydride, reacted for 2 to 5 hours after dropping, controlled by TLC until the reaction was completed, distilled under reduced pressure, dissolved the residue with ethyl acetate, washed twice with 200mL saturated sodium bicarbonate, and evaporated to dryness to obtain 3-acetylamino-5- Bromopyridine 49.57g, yield 89.04%.
[0037] The preparation of second step 3-acetylaminopyridine-5-boronic acid pinacol ester:
[0038] Add 49.5 g (0.23 mol) of 3-acetamido-5-bromopyridine, 58.42 bis(pinacolate)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com