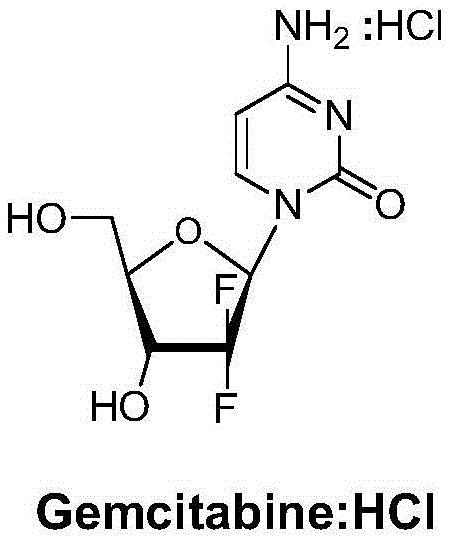
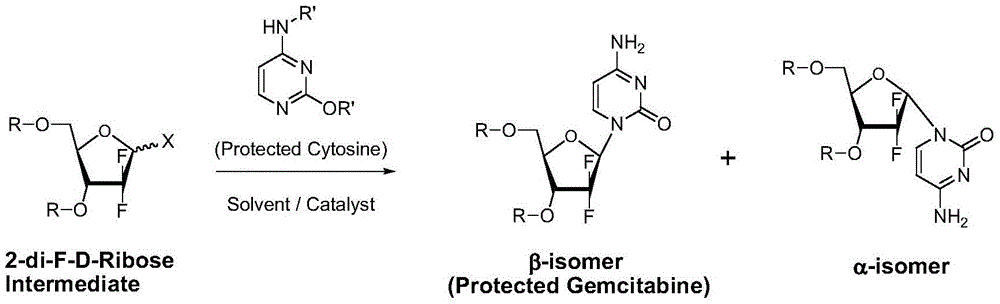
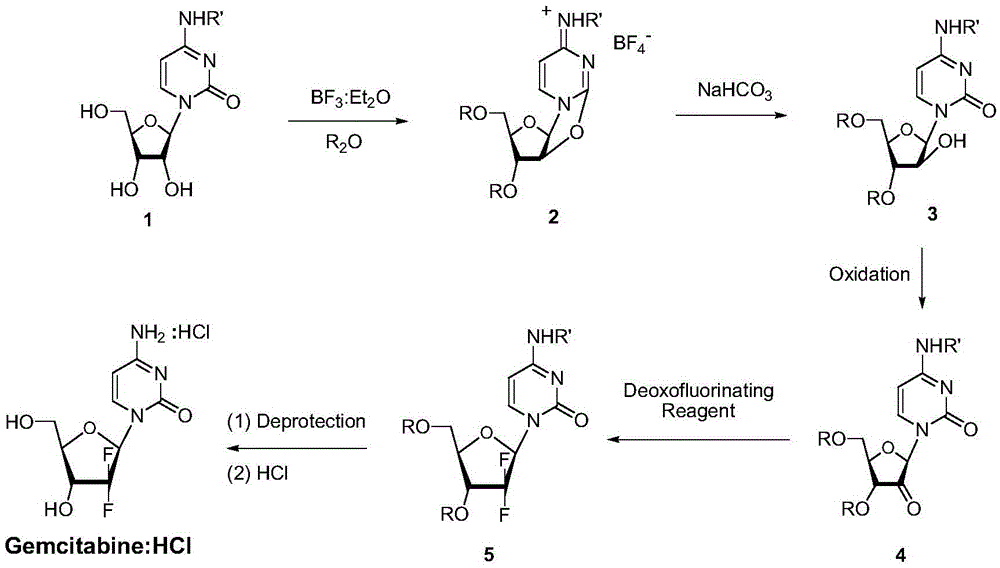
A kind of preparation method of gemcitabine hydrochloride
A technology of gemcitabine hydrochloride and hydrochloric acid, which is applied in the field of preparation of anti-tumor active compound gemcitabine hydrochloride, can solve the problems of not improving and solving α and β isomers, not suitable for large-scale industrial production, and large product loss, so as to eliminate the formation of Possibility of by-product α isomer, high industrial application value, effect of reduction and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 3'-O,5'-O,N 4 - Preparation of tribenzoyl-2,2'anhydrocytidine (II).
[0032] 3'-O,5'-O,N 4 The synthetic route of -tribenzoyl-2,2'anhydrocytidine (II) is as follows:
[0033]
[0034] At room temperature, in the reaction vessel, the N 4 - Benzoylcytidine (I) (347.3g, 1.0mol) was dissolved in acetonitrile (6.0 liters), and boron trifluoride-diethyl ether complex (BF 3 :Et 2O, 246.9ml, 283.9g, 2.0mol), after stirring at room temperature for 30 minutes, heat the reaction at 80°C, and after 1 hour, add benzoyl anhydride (471.7ml, 565.6g, 2.5mol) dropwise, after the addition, in After stirring and reacting at 80°C for 2 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction system was rotary evaporated to dryness, then ground and stirred in diethyl ether (1.6 L) for 10 minutes, and the crude product precipitated out. Filter and dry. The dried crude product was crystallized from isopropanol and ethyl acetate (isopropanol:ethyl a...
Embodiment 2
[0054] N 4 - Preparation of benzoyl 3'-O,5'-O-di-p-chlorobenzoyl-2,2'anhydrocytidine (VI).
[0055] N 4 The synthetic route of -benzoyl 3'-O,5'-O-di-p-chlorobenzoyl-2,2'anhydrocytidine (VI) is shown below:
[0056]
[0057] At room temperature, in the reaction vessel, the N 4 - Benzoylcytidine (I) (347.3g, 1.0mol) was dissolved in acetonitrile (6.0 liters), and boron trifluoride-diethyl ether complex (BF 3 :Et 2 O, 246.9ml, 283.9g, 2.0mol), after stirring at room temperature for 30 minutes, the reaction was heated at 80°C. After 1 hour, p-chlorobenzoic anhydride (737.8g, 2.5mol) was added. After stirring the reaction for 2 hours, HPLC showed that the reaction was complete, and the reaction was stopped. The reaction system was rotary evaporated to dryness, then ground and stirred in diethyl ether (1.6 liters) for 10 minutes, and the crude product precipitated out. Filter and dry. The dried crude product was crystallized from isopropanol and ethyl acetate (isopropanol:e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com