Tapentadol hydrochloride injection and preparation method thereof
A technology of tapentadol hydrochloride and tapentadol, which is applied in the preparation of tapentadol hydrochloride small-volume injection and the field of tapentadol hydrochloride small-volume injection, can solve the problems of slow onset, slow absorption and distribution, Low bioavailability and other problems, to achieve the effect of fast distribution, fast onset and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
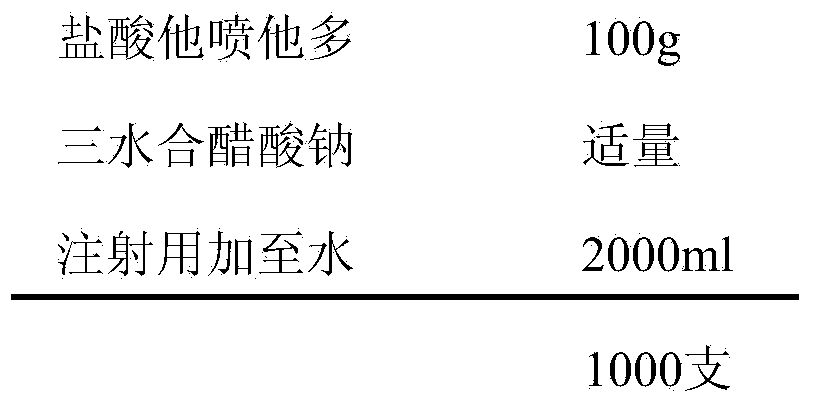
[0029] prescription:
[0030]
[0031] Preparation Process:
[0032] Weigh the prescribed amount of tapentadol hydrochloride, add an appropriate amount of water for injection to dissolve, add 0.6% activated carbon for injection, heat and stir at 50°C-60°C for 30min, filter and decarbonize, cool to room temperature, and add 1mol / L trihydrate Adjust the pH value to 5.5-6.5 with sodium acetate, add water for injection to the full amount, measure the intermediate content and pH value, filter through a 0.22μm microporous membrane after passing the test, fill in 2ml ampoule bottles, fill 2ml in each bottle, and store at 121°C Autoclave for 20 minutes, label, pack, and get the finished product after passing the inspection.
Embodiment 2
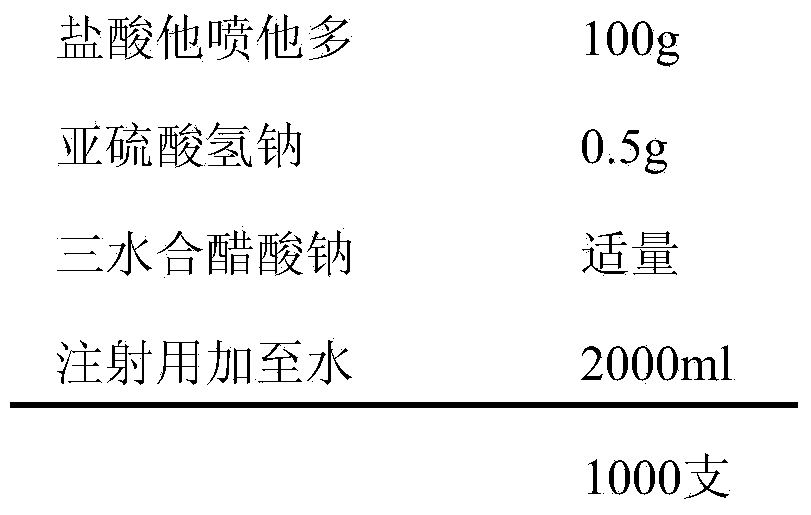
[0034] prescription:
[0035]
[0036] Preparation Process:
[0037] Weigh the prescribed amount of tapentadol hydrochloride, add an appropriate amount of water for injection to dissolve, add the prescribed amount of sodium bisulfite, dissolve, add 0.6% activated carbon for injection needles, keep stirring at 50°C-60°C for 30min, filter and decarbonize , cooled to room temperature, adjusted the pH value to 5.5-6.5 with 1mol / L sodium acetate trihydrate, added water for injection to the full amount, measured the intermediate content and pH value, filtered through a 0.22μm microporous membrane after passing the test, and filled in 2ml ampere In bottles, each bottle is filled with 2ml, sterilized by autoclaving at 121°C for 20 minutes, labeled, packaged, and the finished product is obtained after passing the inspection.
Embodiment 3
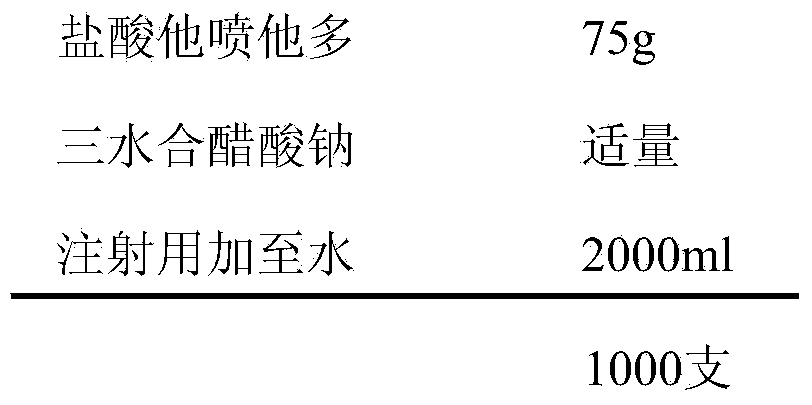
[0039] prescription:
[0040]
[0041] Preparation Process:
[0042] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com