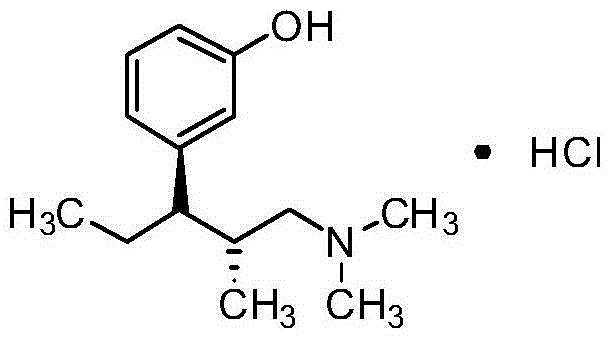
Tapentadol hydrochloride injection and preparation method thereof
A technology for tapentadol hydrochloride and injection, which is applied in the preparation of tapentadol hydrochloride small-volume injection and the field of tapentadol hydrochloride small-volume injection, which can solve the problems of slow absorption and distribution, low bioavailability, and Slow effect and other problems, to achieve the effect of fast distribution, high bioavailability, and fast onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
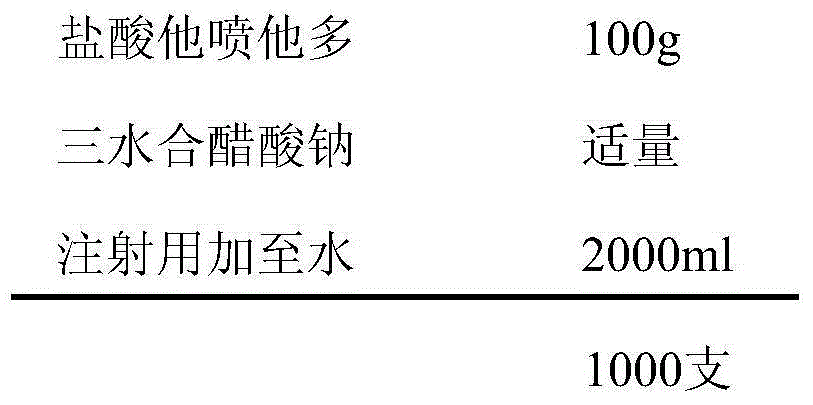
[0030] prescription:
[0031]
[0032] Preparation Process:
[0033] Weigh the prescribed amount of tapentadol hydrochloride, add an appropriate amount of water for injection to dissolve, add 0.6% activated carbon for injection, heat and stir at 50°C to 60°C for 30min, filter and decarbonize, cool to room temperature, and dissolve with 1mol / L trihydrate Adjust the pH value to 5.5-6.5 with sodium acetate, add water for injection to the full amount, measure the intermediate content and pH value, filter through a 0.22μm microporous membrane after passing the test, fill in 2ml ampoule bottles, fill 2ml in each bottle, and store at 121°C Autoclave for 20 minutes, label, pack, and get the finished product after passing the inspection.
Embodiment 2
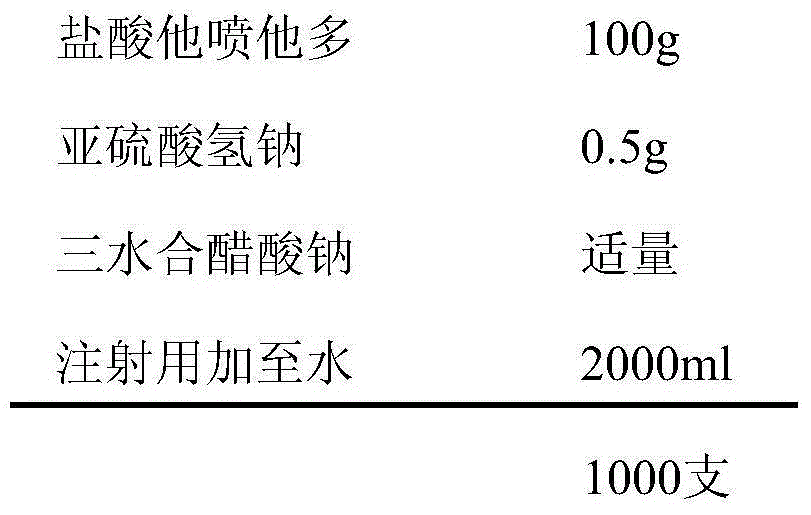
[0035] prescription:
[0036]
[0037] Preparation Process:
[0038] Weigh the prescribed amount of tapentadol hydrochloride, add an appropriate amount of water for injection to dissolve, add the prescribed amount of sodium bisulfite, dissolve, add 0.6% activated carbon for injection needles, keep stirring at 50°C-60°C for 30min, filter and decarbonize , cooled to room temperature, adjusted the pH value to 5.5-6.5 with 1mol / L sodium acetate trihydrate, added water for injection to the full amount, measured the intermediate content and pH value, filtered through a 0.22μm microporous membrane after passing the test, and filled in 2ml ampere In bottles, each bottle is filled with 2ml, sterilized by autoclaving at 121°C for 20 minutes, labeled, packaged, and the finished product is obtained after passing the inspection.
Embodiment 3
[0040] prescription:
[0041]
[0042] Preparation Process:
[0043] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com