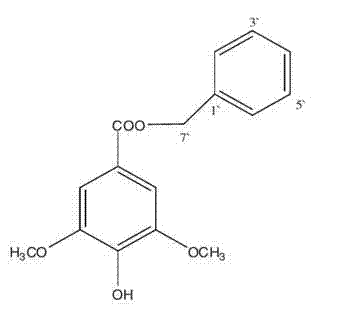
Application of benzyl-4-hydroxy-3,5-dimethoxy benzoate in preparation of drugs for treating or preventing rheumatoid arthritis
A technology of dimethoxybenzoate and benzyl, applied in the field of proteasome-inhibiting compounds for the prevention and treatment or prevention of rheumatoid arthritis, to achieve the effect of preventing or treating rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Immunoprotective effects of proteasome-inhibiting compounds in collagen-induced mouse arthritis animal model
[0022] To construct an animal model of collagen-induced arthritis in mice, and to study the therapeutic effect of proteasome-inhibiting compounds on collagen-induced arthritis (CIA) in mice. Mice were used as experimental animals, 90 SPF grade DBA / 1 mice (provided by Sino-British SIPPR Lab. Animal Ltd, animal production license number: SCXK (Shanghai )2008-0016), male, 7-8 weeks old, weighing 18-22 g, were randomly divided into 6 groups, namely the normal control group, the model control group, and 3 low, middle and high dose groups of the compound (0.4, 0.8, 1.6 mg / kg) and positive drug control group (methotrexate 2 mg / kg). In addition to the normal group, the mouse CIA model was established in each experimental group on day 0 by dissolving chicken cartilage type II collagen (cII) with 0.1 mol / l acetic acid into a 4 mg / ml solution and placing it in a refrige...
Embodiment 2
[0027] In vivo immunoprotective effect of proteasome inhibiting compounds on adjuvanted rat arthritis animal model
[0028] To construct an animal model of adjuvant arthritis in rats, and to study the therapeutic effect of proteasome-inhibiting compounds on Adjuvant arthritis (AA) rats. Rats were used as experimental animals, 90 SPF grade SD rats (provided by Sino-British SIPPR Lab. Animal Ltd, animal production license number: SCXK (Shanghai) 2008 -0016), male, weighing 140 g-160 g, were randomly divided into 6 groups, which were normal control group, model control group, three dose groups of low, medium and high doses of proteasome inhibitory compounds (0.2, 0.4, 0.8 mg / kg) and Positive drug control group (methotrexate 1 mg / kg). In addition to the normal group, on the 0th day, each experimental group established a rat AA model by injecting complete Freund's adjuvant containing inactivated Mycobacterium tuberculosis (H37RA, 10 mg / ml) into the left hindquarters of the rats. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com