Octa-sulfonic phthalocyanine, and preparation method and application thereof
A technology of octasulfonic acid group and disulfonic acid group, which is applied in the field of octasulfonic acid group-substituted phthalocyanine metal complexes and its preparation, can solve the problems of poor biological selectivity, poor stability, easy aggregation, etc., and achieve high gloss Dynamic anti-cancer activity, large molar absorption coefficient, and the effect of improving transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
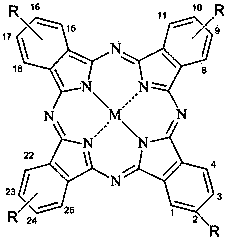
[0036] In the present invention, the preparation method of tetra-a-(6,8-sodium disulfonate base-2-naphthyloxy) phthalocyanine metal complex comprises the following steps:
[0037] (1) Preparation of 3-(6,8-disulfonic acid-2-naphthyloxy) phthalonitrile dipotassium salt: 3-nitrophthalonitrile and 2-naphthol-6,8-di Dipotassium sulfonate was used as a reactant, dimethyl sulfoxide was used as a solvent, in the presence of potassium carbonate and under the protection of nitrogen, the reaction was stirred at room temperature ~45°C for 48~96 hours, monitored by thin layer chromatography, when 3-nitro-o When phthalonitrile is basically consumed, the reaction is terminated, and the target product is purified by solvent method, recrystallization method and extraction method; in the above reaction, 3-nitrophthalonitrile and 2-naphthol-6,8-disulfonic acid disulfide The molar ratio of potassium is 1:1 ~ 1.5, the solvent consumption is that every mmol reactant 3-nitrophthalonitrile needs 2 ~...
Embodiment 1
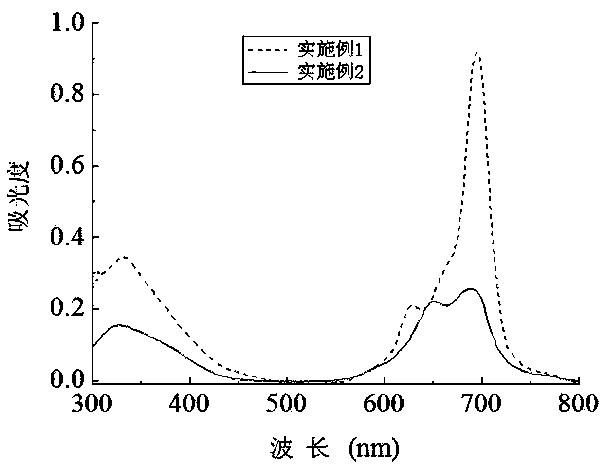
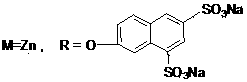
[0051] Synthesis and Physicochemical Properties of 1,8(11), 15(18), 22(25)-tetrakis(6,8-disulfonic acid sodium-2-naphthyloxy)zinc phthalocyanine
[0052]
[0053] Formula 1)
[0054] This compound can also be called tetra-a-(6,8-sodium disulfonate-2-naphthyloxy)zinc phthalocyanine, and its structure is shown in formula (1), wherein:
[0055] .
[0056] (1) Preparation of 3-(6,8-disulfonic acid-2-naphthyloxy) phthalonitrile dipotassium salt: with 3-nitrophthalonitrile (5 mmol) and 2-naphthol-6, Dipotassium 8-disulfonate (5~7.5mmol, preferably 5mmol) was used as reactant, dimethyl sulfoxide (10~25mL, preferably 10mL) was used as solvent, in potassium carbonate (7.5~15mmol, preferably 10mmol), divided into three In the presence of batch addition) and nitrogen protection, the reaction was stirred at room temperature to 60°C (preferably 45°C) for 48 to 96 hours, monitored by thin-layer chromatography, and the reaction was terminated when 3-nitrophthalonitrile was basically c...
Embodiment 2
[0061] Synthesis of 2(3), 9(10), 16(17), 23(24)-tetrakis(6,8-disulfonate sodium-2-naphthyloxy)zinc phthalocyanine
[0062]
[0063] Formula (2)
[0064] This compound can also be called tetrakis-b-(6,8-sodium disulfonate-2-naphthyloxy)zinc phthalocyanine, and its structure is shown in formula (2), wherein:
[0065]
[0066] Substituting equimolar 4-nitrophthalonitrile for 3-nitrophthalonitrile in Example 1, the corresponding peripheral octasulfonic acid group substituted phthalocyanine metal complexes, i.e. four-beta- Zinc (6,8-sodium disulfonate-2-naphthyloxy)phthalocyanine. The structure of the obtained product is the same as that of the phthalocyanine product described in Example 1, except that the position of the substituent is replaced by the β position.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap