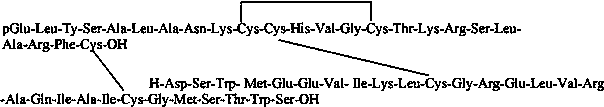Relaxin injection
A technology of peptide injection and relaxin peptide, which is applied in the fields of peptide/protein components, cardiovascular system diseases, non-effective components of polymer compounds, etc., and can solve the problem of inability to achieve high-concentration relaxin injection stability and relaxin injection concentration Problems such as increase and stability decrease, etc., to achieve the effect of being suitable for large-scale industrial production, easy control of process conditions, and improvement of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] Reference Example 1 Preparation of Relaxin Injection (without PEG200 or PEG400)
[0024] 1) Adjust the pH of 100mL water for injection with citrate buffer solution to 5.0, add NaCl to make the solution ionic strength 0.15μ;
[0025] 2) Dissolve 150 mg of relaxin peptide in the solution of step 1), add 35 mg of mannitol, and then add bovine serum albumin to make the concentration 2% (W / V), stir evenly at 3°C for 20 hours, and use lemon Salt buffer solution to adjust the pH to 5.0.
Embodiment 2
[0026] Reference Example 2 Preparation of Relaxin Injection (without bovine serum albumin or human serum albumin)
[0027] 1) Adjust the pH of 100mL water for injection with acetate buffer solution to 5.5, add NaCl to make the solution ionic strength 0.18μ;
[0028] 2) Dissolve 150 mg of relaxin peptide in the solution of step 1), add PEG400 to make the concentration 6% (W / V), stir and mix evenly, leave it at 5°C for 24 hours, and adjust the pH to 5.5 with acetate buffer solution .
Embodiment 3
[0029] Reference Example 3 Preparation of Relaxin Injection (without PEG200, PEG400, bovine serum albumin, human serum albumin)
[0030] 1) Adjust the pH of 100mL water for injection with citrate buffer solution to 5.5, add NaCl to make the solution ionic strength 0.15μ;
[0031] 2) Dissolve 200 mg of relaxin peptide in the solution of step 1), add 50 mg of sorbitol, stir and mix evenly, leave it under stirring at 2°C for 12 hours, and adjust the pH to 5.5 with citrate buffer solution.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap