Carbazolophenanthridine compounds with diaza aromatic fused ring structure and synthesis method thereof
A carbazolophenanthridine and synthesis method technology, which is applied in the field of azaaromatic fused ring compounds and their synthesis, can solve the problems of enhanced interaction force and poor solubility, and achieve improved solubility, simple reaction operation, and easy Promoted app performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
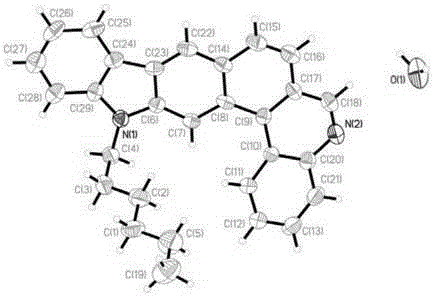
Image
Examples
Embodiment 1
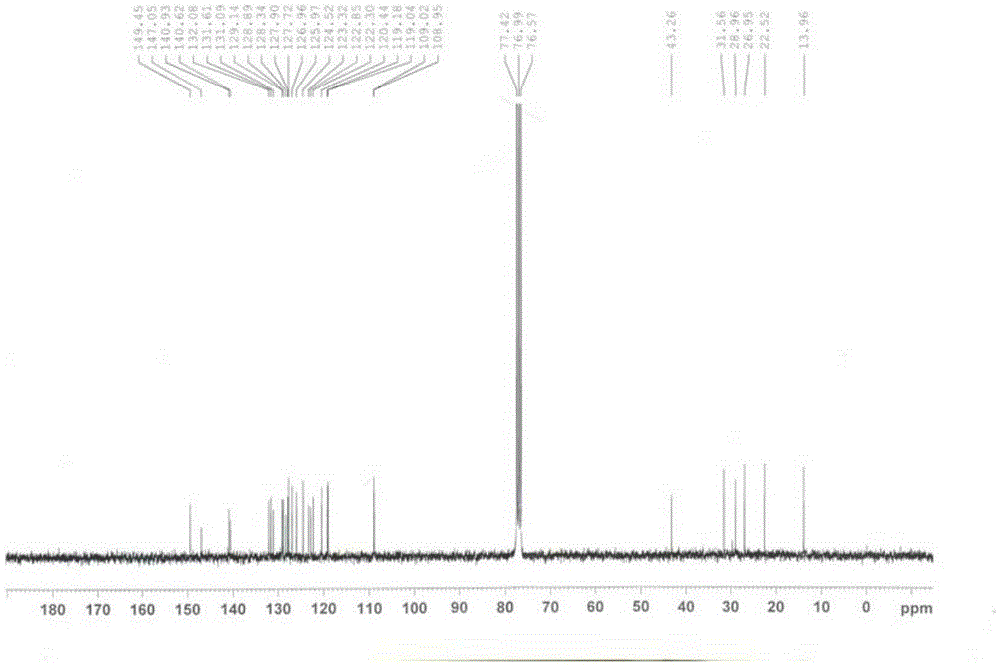
[0064] Example 1: Preparation of 14-hexyl-carbazolophenanthridine (Ia)
[0065]
[0066] Specific steps are as follows:
[0067] (1) Purify the organic solvent benzene by atmospheric distillation, and store it under an inert atmosphere until use;
[0068] (2) Combine trans-9-hexyl-3-(2-quinoline-vinyl)carbazole (Ⅱa, 0.45mmol) and iodine (I 2 , 0.46mmol) into the purified 500ml organic solvent benzene in step (1), stir rapidly while dissolving, after completely dissolving, pass inert gas for 30 minutes;
[0069] (3) Under continuous stirring, add 18ml of propylene oxide into the solution obtained in step (2), so that the concentration of propylene oxide in the solution is 0.51 mol / liter;
[0070] (4) Use a 500W high-pressure mercury lamp to irradiate the solution obtained in step (3) through quartz glass for 10 minutes until the reaction is completed to obtain a crude product;
[0071] (5) The solvent is evaporated in vacuo, and the obtained crude product is dissolved in ...
Embodiment 2
[0077] Embodiment 2: Preparation of 11-bromo-14-hexyl-carbazolophenanthridine I (b)
[0078] The chemical reaction formula is as follows:
[0079]
Embodiment 3
[0080] Example 3: Preparation of 11-bis(trimethylphenyl)boryl-14-hexyl-carbazolophenanthridine I (c)
[0081] The chemical reaction formula is as follows:
[0082]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com