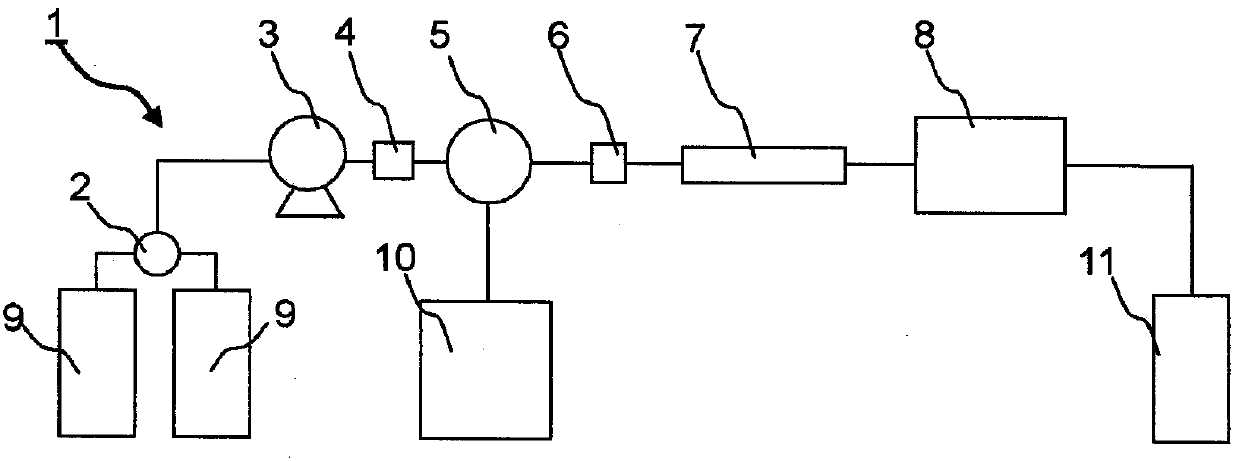
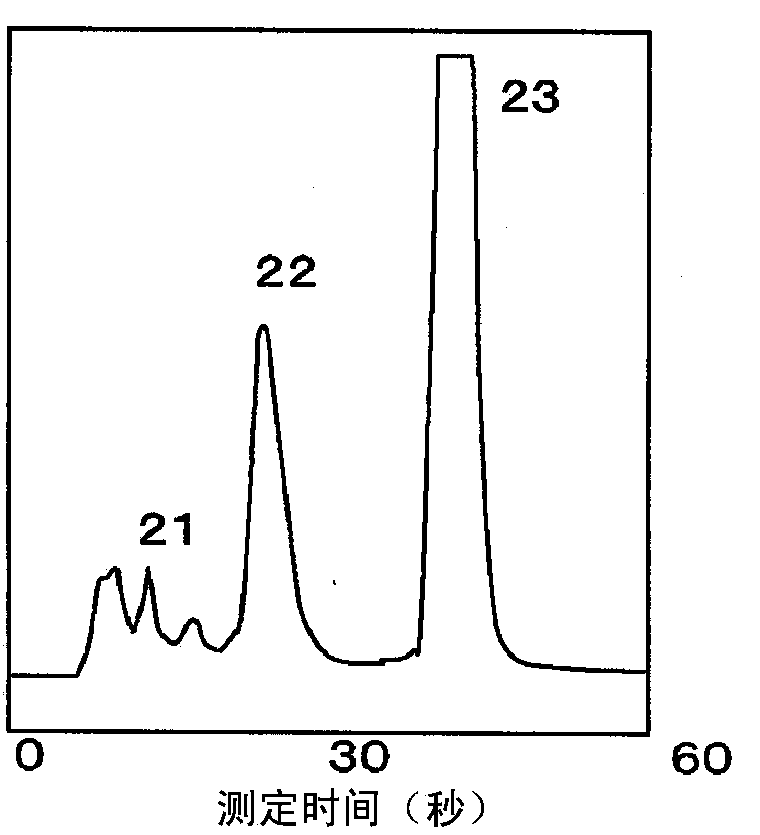
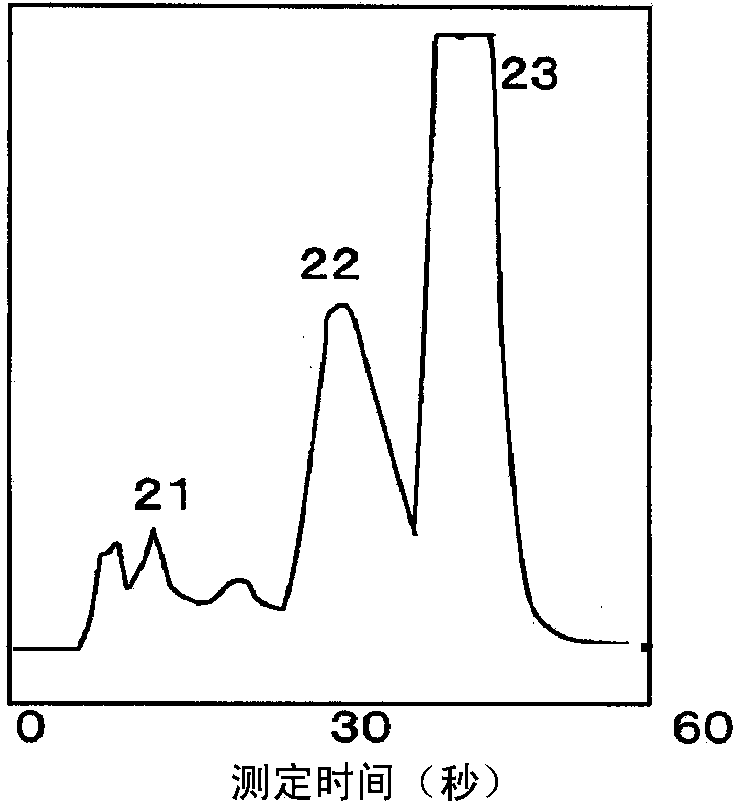
Method for measuring stable hemoglobin a1c using liquid chromatography, and method for simultaneous measurement of stable hemoglobin a1c and abnormal hemoglobin using liquid chromatography
A technology of hemoglobin and liquid chromatography, which is applied in the field of determination of stable hemoglobin A1c, can solve problems such as changes in measured values, increased coating thickness, and clogging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1)
[0124] In Production Examples 1 to 3, column packing materials for measuring hemoglobins having different average particle diameter values were prepared.
[0125] 150 g of tetraethylene glycol dimethacrylate (cross-linkable monomer, manufactured by Shin-Nakamura Chemical Industry Co., Ltd.), 140 g of triethylene glycol dimethacrylate (cross-linkable monomer, manufactured by Shin-Nakamura Chemical Industry Co., Ltd.) , and 2-hydroxy-1,3-dimethacryloyloxypropane (crosslinkable monomer, manufactured by Shin-Nakamura Chemical Industry Co., Ltd.) 60 g of monomer mixture, mix and dissolve benzene peroxide as a polymerization initiator Formyl (manufactured by KISHIDA Chemical Co., Ltd.) 1.0 g was dispersed in 2000 mL of a 5% by weight polyvinyl alcohol (manufactured by Nippon Synthetic Chemicals Co., Ltd., “Gohsenol GH-20”) aqueous solution. While stirring the reaction system at a speed of 350 rpm, it was heated to 80° C. in a nitrogen atmosphere to perform a polymerization reactio...
manufacture example 2)
[0127] A column packing was obtained in the same manner as in Production Example 1 except that the stirring conditions of the reaction system in Production Example 1 were changed to 300 rpm. As a result of measuring the average particle diameter value in the same manner as in Production Example 1, it was 6.4 μm.
manufacture example 3)
[0129] A column packing was obtained in the same manner as in Production Example 1 except that the stirring conditions of the reaction system in Production Example 1 were changed to 250 rpm. The average particle diameter was measured in the same manner as in Production Example 1, and it was 9.5 μm.
[0130] Table 1 shows the average particle diameters of the column fillers obtained in Production Examples 1 to 3 above.
[0131] [Table 1]
[0132]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap